Figures & data
Figure 1. Separation of dimeric and monomeric forms of a recombinant hybrid-IgG/IgA specific for Stx1B. A serum-free culture supernatant (60 ml) of CHO-K1 cells triple transfected with vector constructs for H, L and J chains of the hybrid-IgG/IgA was concentrated and separated on a column of Sephacryl S-300. Elution profile of proteins (A). SDS-PAGE (non-reducing conditions) and immunoblot analysis (probed with anti-α chain) of each fraction from the Sephacryl S-300 column (B). An aliquot (9 μl each) of each fraction (fractions 45 to 57) was analyzed. The positions of molecular weight standards are shown on the left. The left arrow indicates the dimeric form while the right arrow indicates the monomeric one. The result for TEPC 15 (8 ng) is shown as an IgA myeloma protein.
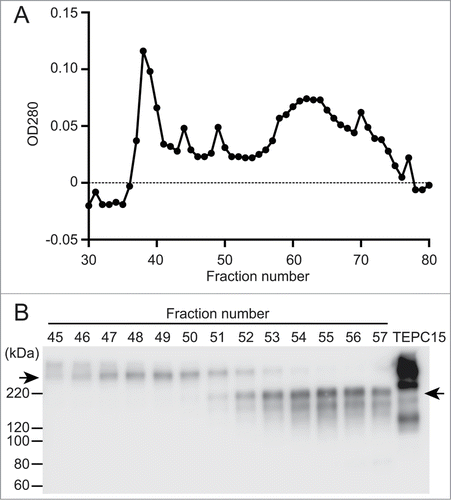
Figure 2. Toxin neutralization by the dimeric hybrid IgG/IgA. Stx1 (5 pg/ml) and varying concentrations (abscissa) of the dimeric hybrid IgG/IgA were incubated for 1 h at 37°C. Each mixture was added to Vero cells (2 × 104), followed by culture for 42 h. Cell viability was determined by an MTT-like assay that measures reducing power of cells with WST-8 as a substrate. The values are relative to that without Stx1 exposure. The bars represent the means ± SD of triplicate determinations.
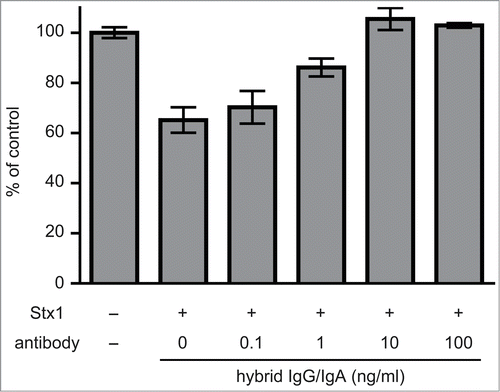
Figure 3. Inhibition of Stx1-induced phosphatidylserine exposure on Ramos cells by the hybrid IgG/IgA. Ramos cells (1 × 106/ml) were incubated with Stx1 (5 pg/ml) in the presence of 10 ng/ml of IgA mAb, IgG1 mAb or the dimeric hybrid IgG/IgA for 5 h. As an early event of apoptosis, phosphatidylserine exposure was detected by annexin V binding (ordinate), while intact plasma membrane permeability was confirmed by the lack of propidium iodide incorporaton (abscissa) by flow cytometric analysis (A). The number in the upper left corner of each panel is the percentage of apoptotic cells. Dose-dependent inhibition of apoptosis (B). Effects of IgA mAb (open circles), IgG1 mAb (open triangles), and the hybrid IgG/IgA (filled circles) are shown.
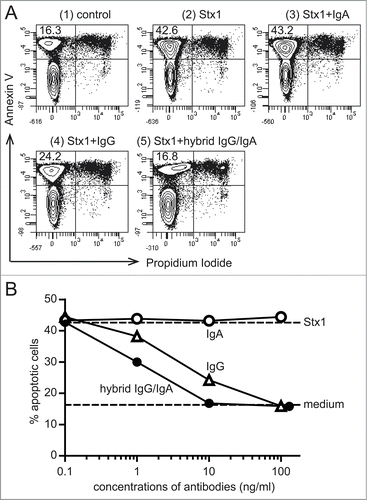
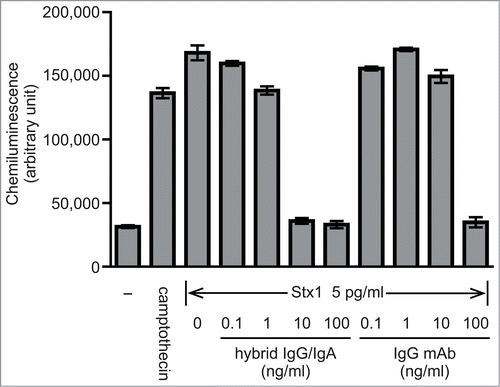
Figure 4. Inhibiton of Stx1-induced caspase-3 activation in Vero cells by the hybrid IgG/IgA. Stx1 (5 pg/ml) was incubated with varying concentrations of the dimeric hybrid IgG/IgA or IgG mAb (D11C6) for 1 h at 37°C. Each mixture was added to Vero cells (2 × 104), followed by culture for 24 h. Activation of caspase-3 was determined as cleavage of the caspase recognition sequence attached to a proluminescent substrate that emits a luminescent signal. Camptothecin was used as a positive control. Data are expressed as the means ± SD of triplicate determinations.