Figures & data
Table 1. Candida BSI and sepsis
Figure 1. Advantages and disadvantages of C. albicans infection models. The most commonly employed C. albicans infection models are immortalized cell culture, primary immune cells, whole blood and mice. Each method bears both limitations and advantages, a thorough knowledge of which can be applied to determining the most suitable model.
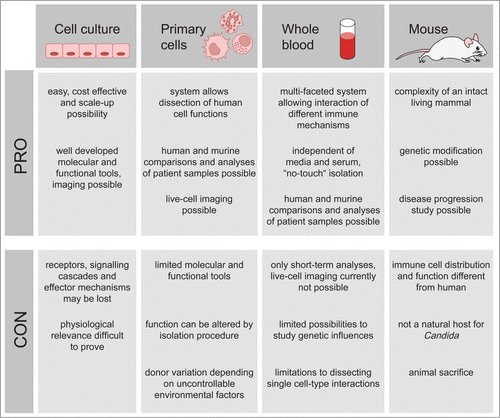
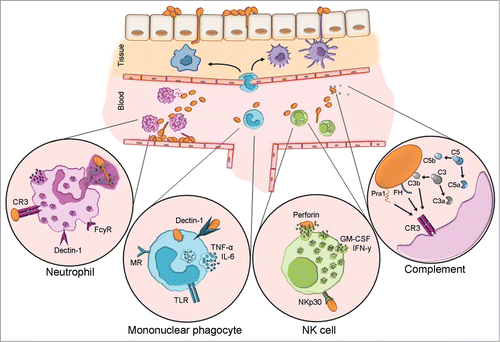
Figure 2. Host innate immune responses to C. albicans blood stream infection. Upon transmigration of skin skin/mucosal barrier and entry to the bloodstream, C. albicans will activate the complement system and encounter circulating and resident leukocytes. Neutrophils are considered the forerunners of innate responses to C. albicans due to their efficient recognition and clearance of the fungus. Complement receptor 3 (CR3) and FCγR are the paramount human neutrophil receptors capable of recognizing C. albicans. Contact to the fungus initiates various signaling cascades, which in turn instigate effector mechanisms e.g. phagocytosis, oxidative burst and neutrophil extracellular trap (NET) formation. Mononuclear phagocytes include circulating monocytes as well as macrophages and dendritic cells residing in various tissues. These cells recognize C. albicans principally via dectin-1 which acts in concert with other pattern recognition receptors. They are a dominant source of IL-6 and TNF-α, both of which can exert direct effects on the fungus and also influence other immune cells. Although NK cells harbor many PRR capable of C. albicans recognition, NKp30 is the principal mediator of NK cell anti-Candida activity. NK cell-released perforin is directly candidacidal. Additionally, NK cells secrete GM-CSF and IFN-γ which both potently modulate other immune cells. Candida is a potent activator of human complement. Complement activation results in opsonization by deposition of C3b and release of anaphylatoxins C5a and C3a which influence immune cell recruitment and effector mechanisms. In addition to C3b, recognition of the fungal protein Pra1 and surface-recruited Factor H, a major regulator of complement activation, mediate recognition by immune cell CR3.