Figures & data
Figure 1 (See previous page). The Rac-FRET mouse is an invaluable tool to assess and quantify Rac1 activity in primary cells (A and B) and in vivo (C and D). (A) Neutrophil harvested from a Rac-FRET mouse migrating toward the chemoattractant N-formyl-methionyl-leucyl-phenyalanine (fMLP) south of the cell. Time series of Rac1 activity was obtained by ratiometric FRET live imaging and illustrated as a heat map of high (yellow to red) and low (blue to green) Rac1 activity. High Rac1 activity was localized to leading edge protrusions (green box) with short-lived bursts at the cell's periphery and at the trailing edge. (B) Maximum Rac1 activity along the longitudinal axis of a neutrophil (blue) migrating toward an fLMP gradient oscillated between the leading (green) and lagging edge (red) illustrated by fitting the experimental data (purple). (C) Intravital FLIM-FRET imaging demonstrated that Cre-mediated loss of APC in intestinal crypts promotes Rac1 activity. Low Rac1 activity in APC wild type mice (left panel) is represented by high fluorescence lifetimes (low FRET-efficiency, green), while loss of APC (right panel) results in high FRET-efficiency (low lifetimes, blue) indicating increased Rac1 activity. Each panel consists of fluorescence images of Rac-FRET expressing crypt cells (blue) and collagen (red, assessed by SHG imaging) on the left and FLIM images on the right. (D) Quantification of Rac1 activity by FLIM-FRET imaging reveals a significant decrease in the fluorescence lifetime upon deletion of APC correlating with an increase in Rac1 activity compared to APC wild type cells (mean± SEM; **P < 0.05). Figure adapted from Johnsson et al. Cell Reports Citation93.
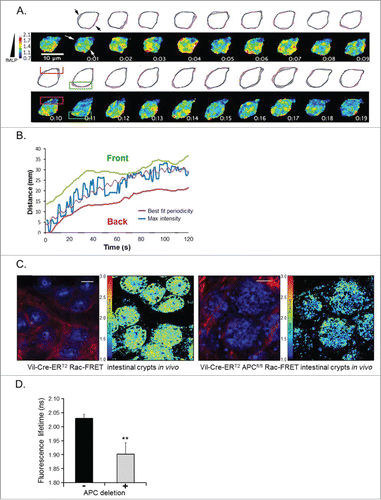
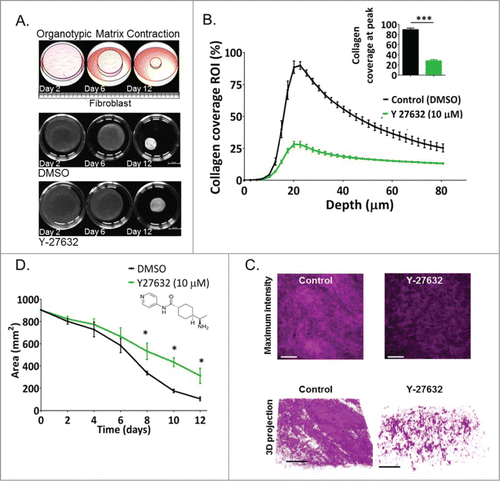
Figure 2. The ROCK inhibitor Y-27632 decreases collagen contraction and crosslinking driven by fibroblasts in 3D organotypic assays. (A) Top panel: Schematic representation and representative pictures of contracting collagen-fibroblast matrices over a 12-day time course. Lower panel: Area of collagen in matrices treated with DMSO (control) and with Y-27632 (10 μM) as a function of time, p* = 0.0268. Scale bar: 10 mm. (B) Collagen coverage (%) quantified by multi-photon SHG imaging as a variable associated with depth (μm) within the 12-day contracted collagen-fibroblast matrices treated with control (DMSO) or with Y-27632 (10 μM) P*** < 0.0001. (C) Maximum intensity projection of SHG signal in representative collagen-fibroblast matrices. D. 3D projection of SHG signal in representative collagen-fibroblast matrices n = 3. Scale bar: 100 μm.