Figures & data
Figure 1. Schematic representation of carotenoid biosynthesis in Chlamydomonas. Mutations in lts1-204 and npq1 lor1 strains are indicated. Norflurazon blocks carotenoid biosynthesis by inhibiting the phytoene desaturase enzyme, the first enzyme of the pathway that converts the colorless phytoene in colored carotenoids in Chlamydomonas.
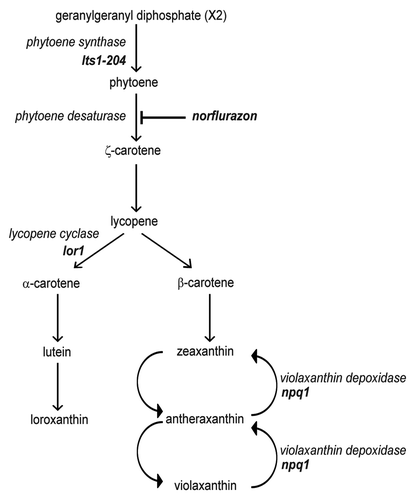
Figure 2. Constitutive autophagic activity in the lts1-204 mutant. (A) Induction and modification of CrATG8 in the lts1-204 mutant. Cells were grown in acetate medium (TAP) to 2 × 106 cells/ml (exponential phase) under dark conditions and soluble extracts were obtained as described in Materials and Methods. Fifteen micrograms of total extracts of Chlamydomonas cells were resolved by 15% SDS-PAGE followed by western blotting with anti-CrATG8 and anti-CrFKBP12 antibodies. The asterisk denotes modified forms of the CrATG8 protein. (B) CrATG8 modification upon dark-to-light transition. Chlamydomonas cells growing exponentially (1 × 106 cells/ml) in TAP under dark conditions were transferred to standard light illumination (20 µE) and samples were taken at different times for its analysis. Cells maintained in dark conditions were used as control. Cell-free extracts were resolved by SDS-PAGE and immunoblotted with anti-CrATG8 and anti-RbcL. Western blot analyses with anti-CrFKBP12 were used as loading controls. (C) Immunolocalization of CrATG8 in wild-type and lts1-204 Chlamydomonas cells. Cells growing exponentially in TAP medium without light were transferred to standard light for 6 h or maintained in dark conditions as a control. Cells were collected and processed for IF microscopy analysis. Scale bar, 8 µm. (D) ROS levels in wild-type and lts1-204 Chlamydomonas strains. Log phase cells growing in TAP medium in the dark were either transferred to standard light for 6 h or maintained in the dark. ROS production was determined as described in Materials and Methods. Values are the means of five independent determinations.
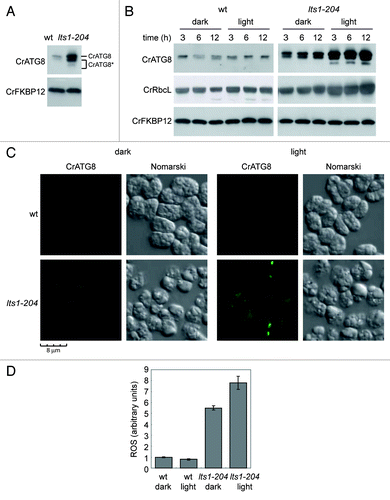
Figure 3. Inhibition of the carotenoid biosynthetic pathway leads to autophagy activation in Chlamydomonas. (A) Induction and modification of CrATG8 by norflurazon (NF) treatment in light or dark growth conditions. Wild-type Chlamydomonas cells in exponential growth phase were treated with 20 µM NF during 6, 12, 24 or 48 h under standard light (left panel) or dark (right panel) conditions. Samples of nontreated cells were taken at the same times and used as control. Fifteen µg of total extracts were resolved by 15% SDS-PAGE followed by western blotting with anti-CrATG8, anti-RbcL and anti-CrFKBP12 antibodies. (B) Immunolocalization of CrATG8 in wild-type cells treated with NF. Chlamydomonas cells growing exponentially under light (right) or dark (left) conditions were treated with NF for 48 h. Nontreated cells were used as control. Cells were collected and processed for IF microscopy analysis. Scale bar, 8 µm. (C) Changes in carotenoid contents in response to NF treatment in light and dark growth conditions. Prominent carotenoids present in wild-type Chlamydomonas cells treated or not with NF for 24 h were determined as described in Materials and Methods. Results are means of three independent experiments. (D) Induction of ROS in response to NF treatment. The content of ROS in TAP-grown cells under light or dark conditions and treated with NF for 24 or 48 h was determined. Untreated cells were used as control. Values are the means of four independent experiments.
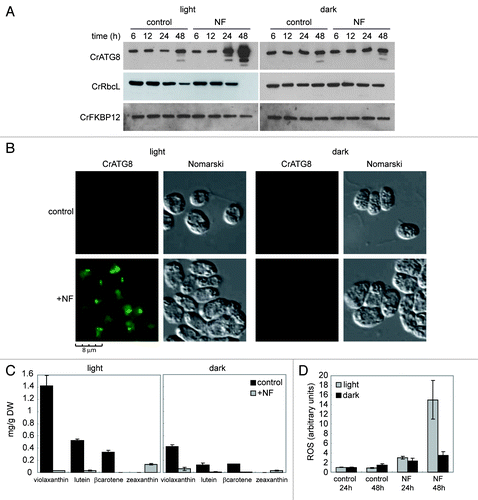
Figure 4. Autophagy is constitutively induced in the npq1 lor1 mutant. (A) Induction and modification of CrATG8 in the npq1 lor1 mutant. Wild-type and npq1 lor1 strains were grown to log phase in TAP medium in dim light conditions. The abundance of CrATG8 was analyzed by western blot using CrFKBP12 as loading control. Fifteen micrograms of total extracts were loaded per lane. (B) High light stress activates autophagy. Wild-type, npq1, lor1 and npq1 lor1 strains were grown in TAP medium to exponential growth phase under dim light and then shifted to high light (1200 µE m−2 s−1). Samples were taken at the indicated times and processed for western blot analysis with the indicated antibodies. Cells maintained in dim light were used as control. (C) Immunolocalization of CrATG8 in wild-type and npq1 lor1 cells under dim light or exposed to high light stress for 6 h. Scale bar, 8 µm. (D) ROS levels in wild-type and npq1 lor1 cells grown to log phase in dim light or shifted to high light for 6 h. Values are the means of four independent determinations.
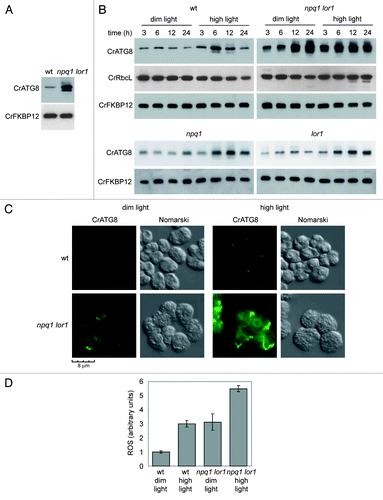
Figure 5. Inhibition of photosynthetic electron flow does not activate autophagy in Chlamydomonas. (A) Wild-type Chlamydomonas cells were grown in TAP medium to log phase under standard light conditions. The photosynthetic inhibitors DCMU (10 µM) or DBMIB (2 µM) were added and samples were taken at the indicated times and processed for western blot analysis. (B) Effect of MV on photosynthesis. Exponentially growing cells were treated with MV (1 µM) in the presence or in the absence of light and samples were collected at different times of treatment. Untreated cells were used as control. The abundance of CrATG8 and RbcL proteins in total extracts was analyzed by western blotting using CrFKBP12 as loading control. (C) Immunofluorescence analysis of CrATG8 in wild-type cells treated with 1 µM MV for 8 h under light and dark conditions. Scale bar, 8 µm. (D) ROS levels in MV treated cells. Cells growing exponentially under light or dark conditions were treated with 1 µM MV for 8 h and ROS levels were determined. Values are the means of five independent experiments. (E) Activation of autophagy by H2O2. CrATG8 abundance was analyzed in light (L) and dark (D) grown cells treated or not (control) with 1 mM H2O2 for 8 h.
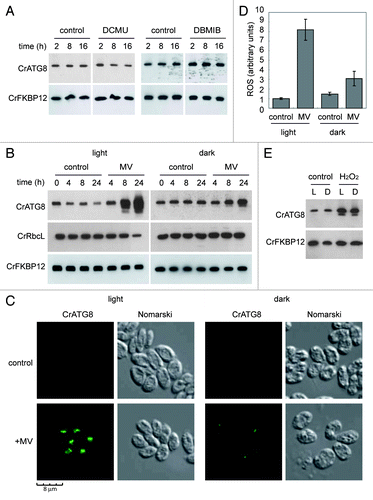
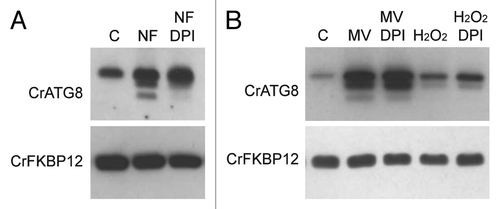
Figure 6. Inhibition of NADPH activity partially suppressed autophagy induction upon carotenoid deficiency. (A) Wild-type Chlamydomonas cells were grown in TAP medium to log phase under standard light conditions and treated with 20 µM NF for 24 h to inhibit carotenoid deficiency. DPI (20 µM) or drug vehicle (C) was then added for 4 h to inhibit NADPH oxidase activity. Autophagy was monitored by western blot analysis with anti-CrATG8 antibody. (B) Oxidative stress was induced in wild-type Chlamydomonas cells with 1 µM MV or 1 mM H2O2 for 8 h, and then DPI (20 µM) or drug vehicle (C) was added to inhibit NADPH oxidase activity. Cells were collected and processed for western blot analysis.