Figures & data
Figure 1. Autophagy in mammalian cells. After induction of autophagy by the inhibition of MTOR in response to various stimuli, the ULK complex is activated and translocated to the rough ER followed by subsequent recruitment of the class III PtdIns3K complex (which includes BECN1) and the generation of its substrate PtdIns3P. Effector proteins (e.g., ZFYVE1, WIPIs) are then recruited to the ER and eventually result in the formation of a phagophore/omegasome, which develops into the autophagosome. Next, cargo is recruited to the autophagic machinery possibly via a ligand-receptor-scaffold complex that ultimately interacts with LC3-II. Two ubiquitin-like conjugation systems (ATG12–ATG5 and LC3–PE) are required for the expansion of the phagophore and completion of the autophagosome. Finally, the maturation of autophagosomes takes place when they fuse with acidic lysosomes to form autolysosomes where the cargo is degraded by lysosomal enzymes.
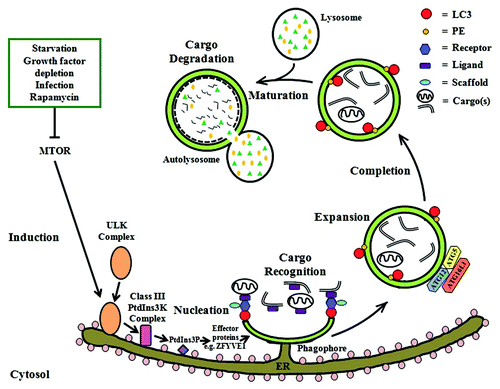
Table 1. Association of host cell autophagic processes with different pathogenic bacteria
Table 2. Host and bacterial factors involved in H. pylori- associated autophagic processes and the intracellular survival of H. pylori
Table 3. Demonstration for H. pylori-containing autophagosomes in different host cell types
Figure 2.H. pylori-associated autophagy in gastric epithelial cells. Upon induction of autophagy by H. pylori in gastric epithelial cells, internalized bacteria are able to multiply inside the autophagosome and are degraded after fusion of the autophagosome with a lysosome. H-pylori-induced autophagy seems to depend on ATG5 and ATG12. The miRNA MIR30B impairs autophagy via downregulation of ATG12 and BECN1. VacA can induce autophagy after internalization mediated by LRP1/other unknown receptors and is degraded within the autophagosome. Acute exposure to VacA induces formation of autophagosomes that results in the degradation of VacA and might contribute to bacterial clearance, whereas chronic exposure to VacA results in the accumulation of SQSTM1 and formation of defective autolysosomes, which lack CTSD. The latter has been proposed to impair cargo degradation and contribute to intracellular survival of H. pylori.
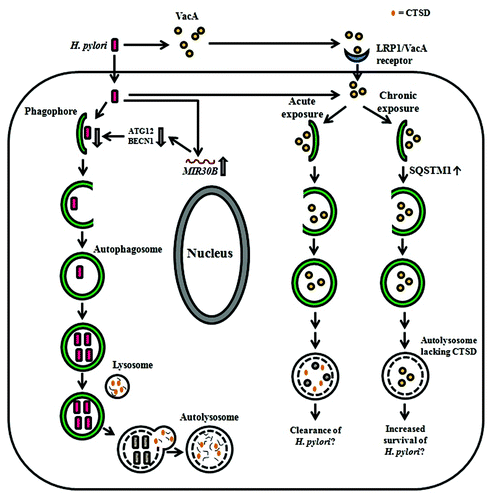
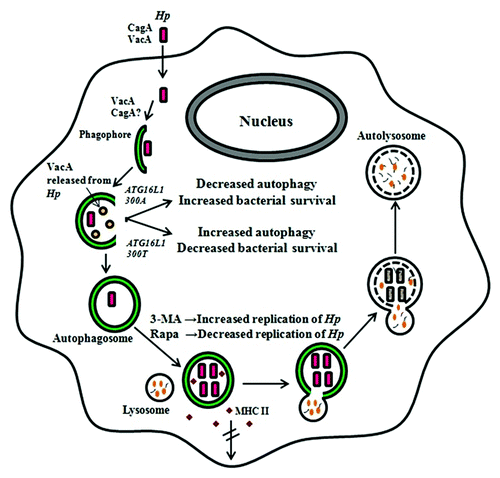
Figure 3.H. pylori-associated autophagy in professional phagocytes. In professional phagocytes such as monocytes, macrophages and BMDCs, internalized bacteria are able to multiply inside the autophagosome. VacA and probably CagA are involved in this process. Bacteria are eventually degraded in the autophagic vesicles. Bacteria inhibit the transport of major histocompatibility complex class II molecules to the cell membrane in wild-type, but not in TLR2- and TLR4-deficient BMDCs. In PMBCs, the ATG16L1300A risk variant for Crohn disease is associated with decreased autophagy, leading to increased bacterial intracellular survival and the individual’s increased susceptibility to H. pylori infections. The events depicted however do not discount the possibility that H. pylori induces LC3-associated phagocytosis (LAP) rather than canonical autophagic pathways. 3-MA, 3-methyladenine; Hp, H, pylori; MHC, major histocompability complex; Rapa, rapamycin; the double-crossed arrow indicates blocking.