Figures & data
Table 1. Dose escalation patient characteristics
Table 2. Adverse events
Table 3. RECIST response in evaluable patients
Figure 1. Antitumor activity of temsirolimus and hydroxychloroquine. (A) Serial contrast CT (CT) scans of the chest and abdomen in a patient with rapidly progressive melanoma treated with temsirolimus and HCQ. Orange outlines: tumor. (B) Serial [18]-fluordeoxy glucose positron emission tomography (FDG-PET) scans of a melanoma patient with massive tumor burden at baseline, who was able to maintain performance status by achieving stable disease on temsirolimus and hydroxychloroquine. Black signal indicates FDG-avid tumor.
![Figure 1. Antitumor activity of temsirolimus and hydroxychloroquine. (A) Serial contrast CT (CT) scans of the chest and abdomen in a patient with rapidly progressive melanoma treated with temsirolimus and HCQ. Orange outlines: tumor. (B) Serial [18]-fluordeoxy glucose positron emission tomography (FDG-PET) scans of a melanoma patient with massive tumor burden at baseline, who was able to maintain performance status by achieving stable disease on temsirolimus and hydroxychloroquine. Black signal indicates FDG-avid tumor.](/cms/asset/652de648-003b-48a9-b7d4-79cd955de138/kaup_a_10929119_f0001.gif)
Figure 2. Pharmacodynamic effects of temsirolimus and hydroxychloroquine on autophagic vacuole accumulation in peripheral blood mononuclear cells (PBMC). (A) Mixed-effects model of mean ± SD autophagic vacuoles (AVs)/cell. *P < 0.05. (B) Representative electron micrographs from a patient treated with TEM and TEM + HCQ 600 mg/po bid. Arrows, AVs; scale bar: 2 µm.
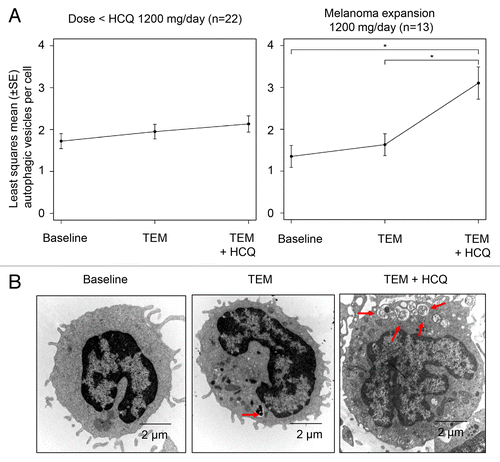
Figure 3. Therapy-associated autophagic vacuole accumulation in serial tumor biopsies from melanoma patients. Representative electron micrographs of a melanoma cell from 2 different patients (A and B) at the indicated timepoints. Dotted blue line: border of cytoplasmic membrane of 1 tumor cell. Red arrows, autophagic vacuoles. Yellow arrow, mitochondria.
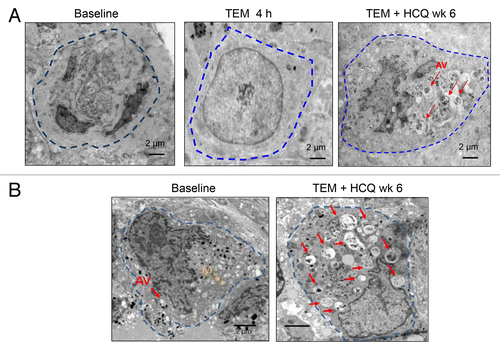
Figure 4. Changes in FDG-PET uptake in patients treated with temsirolimus and HCQ. (A) Serial FDG-PET images in a patient with metastatic melanoma. Arrow: central necrosis. (B–D) Comparison of FDG-PET parameters in patients with no clinical benefit (RECIST measurements > 0) or clinical benefit (RECIST measurement ≤ 0). (B) SUVmax normalized to baseline. (C) Tumor volume normalized to baseline. (D) Partial volume corrected metabolic volumetric product (cMVP); *P < 0.05.
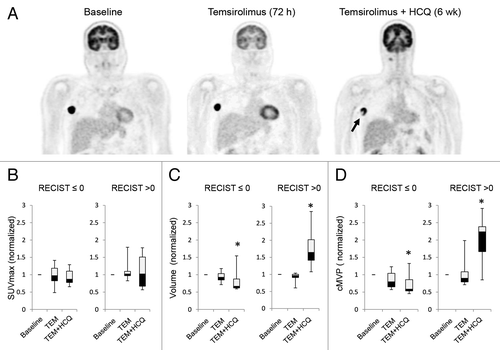
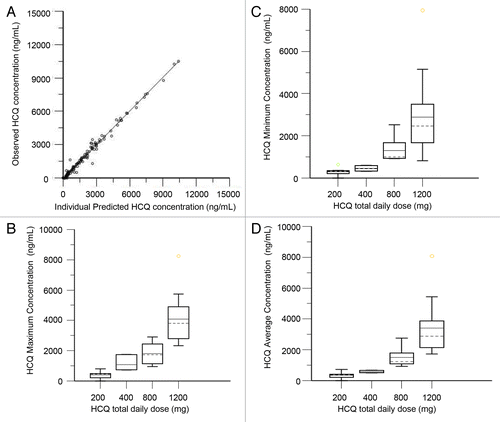
Figure 5. Pharmacokinetic analysis of HCQ in patients receiving temsirolimus and HCQ. (A) Observed vs. individually predicted concentrations of HCQ based on the population PK model. (B) Estimated peak concentrations (Cmax). (C) Estimated trough concentrations (Cmin). (D) Estimated average concentrations (Cavg).