Figures & data
Figure 1. Autophagy is not required to sustain muscle contraction during physical activity. (A) Immunoblot for MAP1LC3A and SQSTM1 proteins on muscle extracts from inducible atg7f/f mice after tamoxifen treatment. (B) Histogram showing the mean maximum distance ran to exhaustion by atg7f/f and atg7−/− mice during acute concentric exercise (n = 16 each genotype). (C) Mean distance covered by females (n = 5 each genotype) and (D) males (n = 11 each genotype) after concentric exercise.
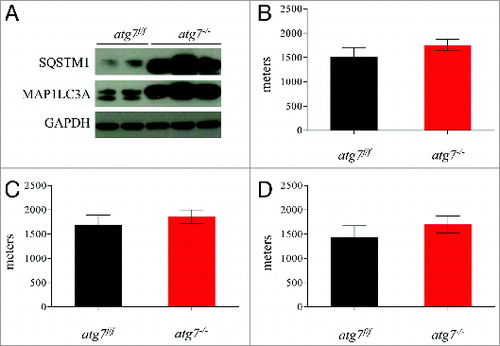
Figure 2. Autophagy is required for eccentric exercise. (A) Representative immunoblots and quantification histograms for MAP1LC3A and SQSTM1 proteins on muscle extracts from inducible atg7f/f mice before and after eccentric exercise (n = 7 each conditions). (B and C) Histograms showing the mean maximum distance ran to exhaustion by atg7f/f and atg7−/− females and males (B) during an acute bout of eccentric exercise (n = 10 males each genotype and n = 10 females each genotype, *P < 0.05), (C) during 3 consecutive d of eccentric exercise (n = 10 males each genotype and n = 10 females each genotype, **P < 0.01; *P < 0.05). (D) Representative hematoxylin and eosin staining of tibialis anterior (TA) muscle cross sections from exercised atg7f/f and atg7−/− animals. No major morphological alterations such as inflammation, center-nucleated fibers are present in exercised muscles of both genotypes. (E) Representative images of IgG staining of cross-sections from exercised TA of atg7f/f and atg7−/− mice. No significant membrane permeabilization was found in either genotype after 3 d of eccentric exercise.
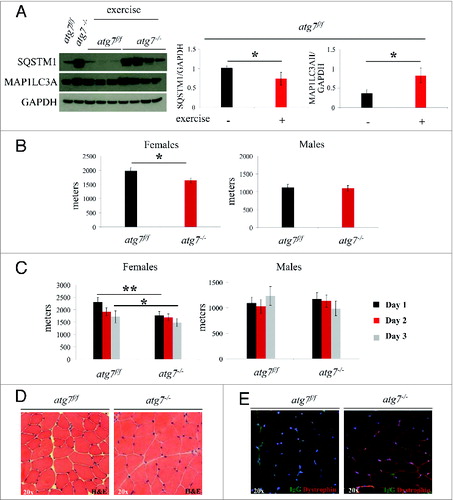
Figure 3. Autophagy is not required for the phosphorylation of PRKAA1. (A) Representative immunoblots from exercised atg7f/f and atg7−/− females. (B) Histograms representing the densitometric quantification of immunoblots in (A). No significant differences in protein expression were observed (n = 3 each genotype).
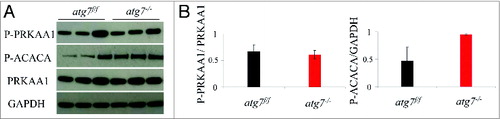
Figure 4. Autophagy inhibition does not impair glucose homeostasis. (A and B) Histograms representing blood concentrations of metabolites in atg7f/f and atg7−/− animals. Analyses were done before and after 3 d of eccentric exercise in (A) males (n = 6 atg7f/f, n = 4 atg7−/−), and (B) females (n = 6 atg7f/f, n = 6 atg7−/−).
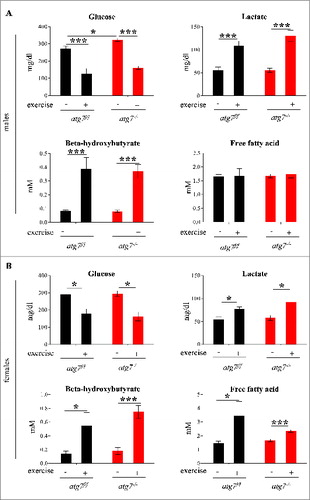
Figure 5. Autophagy inhibition leads to accumulation of dysfunctional mitochondria and increase of oxidative stress during eccentric contraction. (A and B) Mitochondrial membrane potential as measured by TMRM fluorescence in isolated FDB muscle fibers from atg7f/f (top) and atg7−/− (bottom) female mice, (A) pre-exercise and (B) postexercise. Oligomycin (Olm) and the protonophore FCCP were added at the indicated time points. The percentage of depolarized fibers is shown on the bottom of the graphs. Fibers were considered depolarized if TMRM fluorescence decreased by 10% or more of the initial value following the addition of Olm. Each trace represents the TMRM fluorescence of a single fiber. (C) Overall protein carbonylation in exercised atg7f/f and atg7−/− muscles. Left panel: A representative immunoblot for carbonylated proteins. Right panel: Densitometric quantification of the carbonylated proteins. Postexercised atg7−/− mice show higher protein carbonylation than atg7f/f (n = 5 each genotype, *P < 0.05). (D) Mitochondrial ROS production. Mt-roGFP1 fluorescence was measured in single fibers of atg7f/f and atg7−/− (n = 3 each condition, P < 0.05).
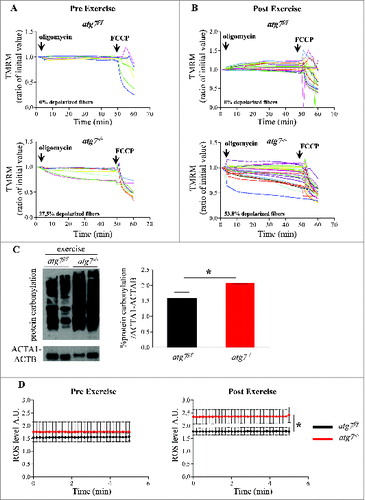
Figure 6. NAC treatment impairs physical performance and mitochondria function in atg7f/f mice. (A) Mean maximal running distance after 1 (left) and 3(right) d of eccentric exercise with and without NAC treatment in atg7f/f and atg7−/− females (NAC-treated n = 6 atg7f/f, n = 4 atg7−/−). NAC treatment did not improve atg7−/− but rather worsened atg7f/f physical performance. (B) TMRM analysis of atg7f/f females pre-exercise (top) and postexercise (bottom) after NAC treatment. Mitochondrial ability to maintain membrane potential is compromised by prolonged antioxidant treatment (n > 15 per condition).
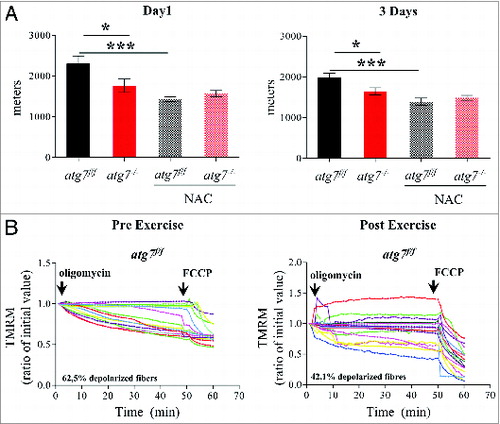
Figure 7. Mitochondria targeted antoxidant Mito-TEMPO impairs the physical performance and mitochondria function of atg7f/f mice. (A) Mean maximal running distance after 1 (left) and 3 (right) d of eccentric exercise with and without Mito-TEMPO treatment in atg7f/f and atg7−/− females (Mito-TEMPO-treated n = 3 atg7f/f, n = 3 atg7−/−). Mito-TEMPO treatment worsened atg7f/f physical performance. (B and C) TMRM analysis of atg7f/f females pre-exercise (B) and postexercise (C) after Mito-TEMPO treatment. Mitochondrial ability to maintain membrane potential is compromised by prolonged antioxidant treatment (n > 15 per condition). (D) Representative immunoblots for the activation of PRKAA1 and ACACA in control and exercised atg7f/f and atg7−/− females, following NAC treatment. (E) Histogram of the densitometric quantification of phospho-PRKAA1 corrected for its total content (n = 3 each condition, P < 0.05).
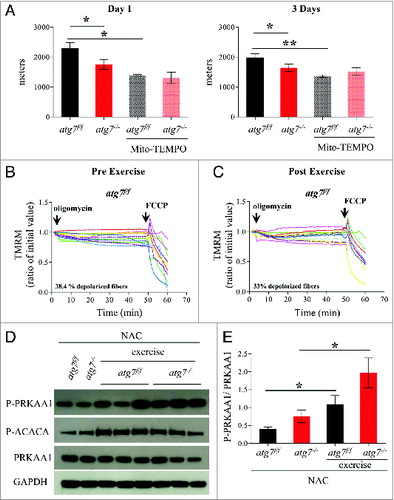
Figure 8. NAC treatment reduces basal autophagy in atg7f/f mice. (A) Representative western blots for SQSTM1 and MAP1LC3A-I/MAP1LC3A-II pre-exercise and postexercise in atg7f/f mice in the presence or absence of NAC. (B and C) Histograms representing the densitometric quantification of (B) MAP1LC3A-II and (C) SQSTM1 (n = 5 each condition, P < 0.05). (D) Representative immunoblots showing the presence of SQSTM1, MAP1LC3A-II, BNIP3, PARK2, COX4I1/COXIV on isolated mitochondria from pre-exercised and postexercised atg7f/f muscles in the presence or absence of NAC. GAPDH immunoblot indicates the purity of the enriched mitochondrial fraction.
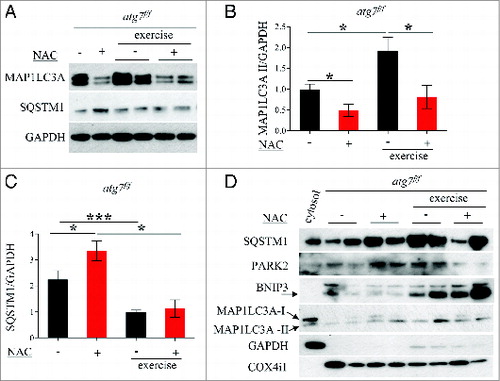
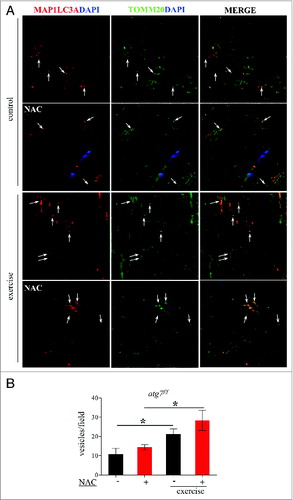
Figure 9. Exercise activates mitophagy in control and NAC-treated mice. (A) Representative confocal images of immunofluorescence staining for MAP1LC3A (red) and the mitochondrial protein TOMM20 (green) of longitudinal cryosections of pre-exercised and postexercised in atg7f/f animals with or without NAC treatment. (B) Quantification of MAP1LC3A and TOMM20 double-positive vesicles (n = 7 each condition, *P < 0.05).