Figures & data
Figure 1. Schematic comparison of the fate of C-terminal versus N-terminal GFP fused to Atg8. (A) If GFP is fused to the C terminus of Atg8 (or LC3), it is removed when Atg4 hydrolyzes the bond between the penultimate glycine residue and arginine. The result is free GFP (with an additional arginine) that does not serve as a marker for the phagophore or autophagosome. However, a C-terminal fusion can be used to monitor Atg4 activity. (B) If GFP is fused to the N terminus of Atg8 (LC3), it remains attached to the protein until vacuolar (lysosomal) delivery; Atg8 is degraded relatively rapidly compared to GFP, so the generation of free GFP in the vacuolar/lysosomal lumen can be used to monitor autophagy activity. Furthermore, the stable GFP-Atg8/LC3 chimera can be used to follow the phagophore or autophagosome. Although the schematic refers to yeast Atg8, essentially the same hold true for LC3 and its related family members.
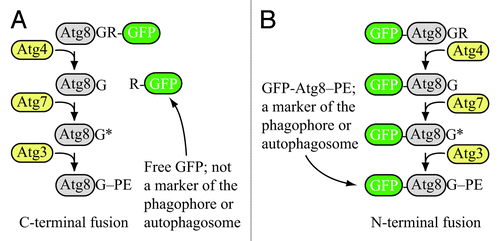
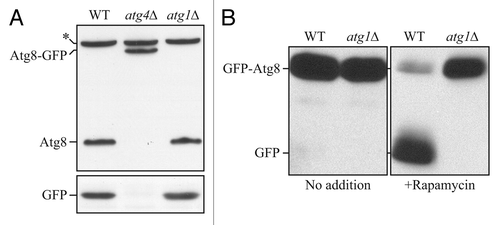
Figure 2. Atg8-GFP cannot be used to monitor autophagic flux. (A) Wild-type (WT), atg4Δ or atg1Δ cells expressing Atg8-GFP were grown to mid-log phase and protein extracts were examined by western blot with antisera to Atg8 or GFP. The asterisk indicates a nonspecific band. With a C-terminal fusion, GFP is cleaved from Atg8 without shifting to autophagy-inducing conditions, and even in an autophagy-defective mutant; however, the Atg8-GFP construct can be used to monitor Atg4 activity. This figure was modified from data previously published in reference Citation1, and is reproduced by permission of the American Society for Cell Biology, copyright 2001. (B) WT or atg1Δ cells expressing GFP-Atg8 were grown to mid-log phase and divided into aliquots; one set of samples was treated with rapamycin and protein extracts were examined by western blot with antiserum to GFP. In this case, free GFP is not generated prior to autophagy induction or in an autophagy-defective mutant. Thus, the N-terminal fusion of GFP can be used to monitor autophagy induction, and also serves as a marker for the phagophore and autophagosome (as well as Cvt or autophagic bodies if breakdown is prevented in the vacuolar lumen, for example, by the addition of PMSF or the use of a mutant such as pep4Δ). This figure was modified from data previously published in reference Citation2, and is reproduced by permission of the American Society for Cell Biology, copyright 2007.