Figures & data
Table 1. Existing calcium orthophosphates and their major propertiesCitation29,Citation30
Figure 1. Simplified schematic of the phosphorus cycle from apatitic igneous rock to phosphorite sedimentary rock through chemical or physical weathering. Life forms accumulate soluble phosphorus species and can produce apatite through biomineralization. Reprinted from reference Citation42 with permission.
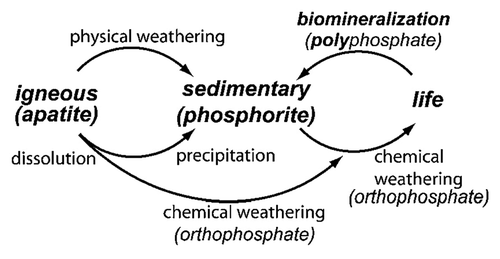
Figure 2. Polycrystalline (A) and single-crystalline (B) FA of a geological origin. The single crystal has a gray-green color due to incorporated ions of transition metals.
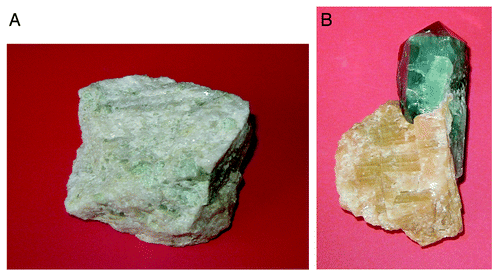
Table 2. Comparative composition and structural parameters of inorganic phases of adult human calcified tissues. Due to the considerable variation found in biological samples, typical values are given in these casesCitation26,Citation32
Figure 3. Phase diagram of the system CaO-P2O5 (C = CaO, p = P2O5) at elevated temperatures. Here: C7P5 means 7CaO·5P2O5; other abbreviations should be written out in the same manner. Reprinted from references Citation110 and Citation111 with permission.
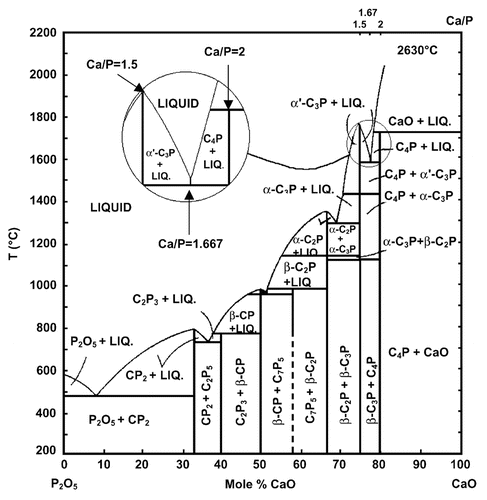
Table 3. Crystallographic data of calcium orthophosphatesCitation27,Citation112,Citation113
Figure 4. pH variation of ionic concentrations in triprotic equilibrium for phosphoric acid solutions. Reprinted from reference Citation116 with permission.
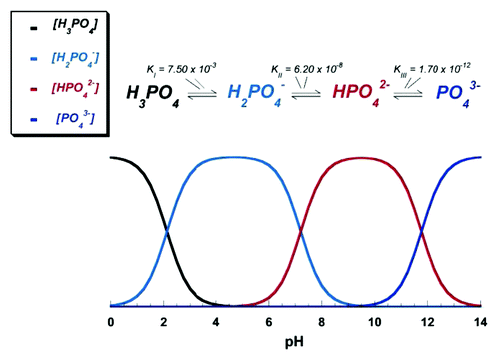
Figure 5. Various calcium orthophosphates obtained by neutralizing of orthophosphoric acid. Ca/P are reported in the figure. The solubility of calcium orthophosphates in water decreases drastically from left to right, HA being the most insoluble and stable phase. Reprinted from reference Citation117 with permission.
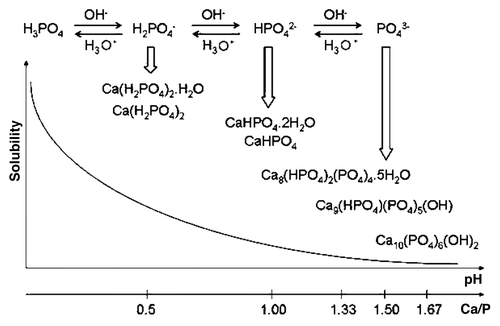
Figure 6. Top: a 3D version of the classical solubility phase diagrams for the ternary system Ca(OH)2-H3PO4-H2O. Reprinted from reference Citation118 with permission. Middle and bottom: solubility phase diagrams in two-dimensional graphs, showing two logarithms of the concentrations of (a) calcium and (b) orthophosphate ions as a function of the pH in solutions saturated with various salts. Reprinted from reference Citation119 with permission.
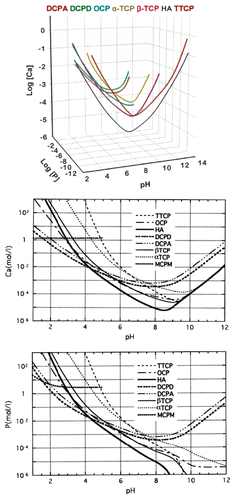
Figure 7. A model of ACP structure. Reprinted from reference Citation278 with permission.
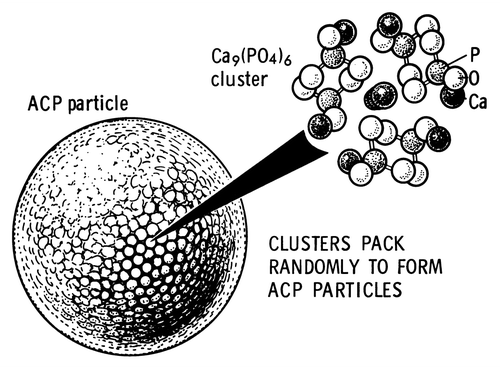
Figure 8. A biomimetically grown aggregate of FA that was crystallized in a gelatin matrix. Its shape can be explained and simulated by a fractal growth mechanism. Scale bar: 10 µm. Reprinted from reference Citation420 with permission.
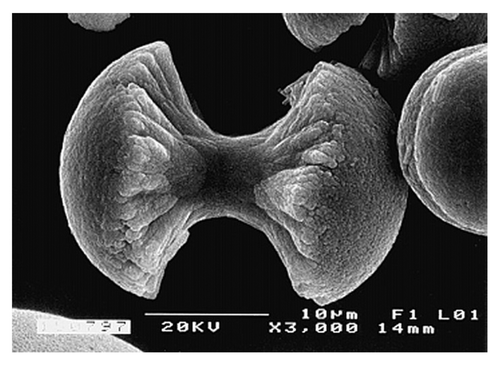
Figure 10. General structure of a mammalian bone. Other very good graphical sketches of the mammalian bone structure are available in references Citation88 and Citation508.
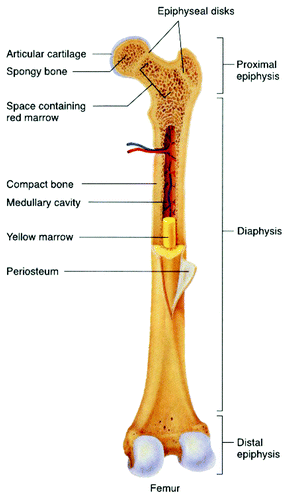
Figure 14. Scanning electron micrograph of the forming enamel of a continuously growing rat incisor showing ordered rods of calcium orthophosphates. Scale bar: 10 µm. Reprinted from reference Citation103 with permission.

Figure 9. Left: crystal structure of a biological apatite. Powder X-ray diffraction patterns (center) and infrared spectra (right) of human enamel, dentine and bone. Reprinted from reference Citation508 with permission.
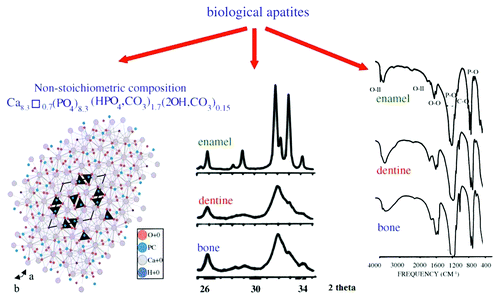
Figure 11. The seven hierarchical levels of organization of the zebrafish skeleton bone. Level 1: Isolated crystals and part of a collagen fibril with the triple helix structure. Level 2: Mineralized collagen fibrils. Level 3: The array of mineralized collagen fibrils with a cross-striation periodicity of nearly 60–70 nm. Level 4: Two fibril array patterns of organization as found in the zebrafish skeleton bone. Level 5: The lamellar structure in one vertebra. Level 6: A vertebra. Level 7: Skeleton bone. Reprinted from reference Citation552 with permission. Other good graphical sketches of the hierarchical structure of bones are available in references Citation104, Citation553 and Citation554.
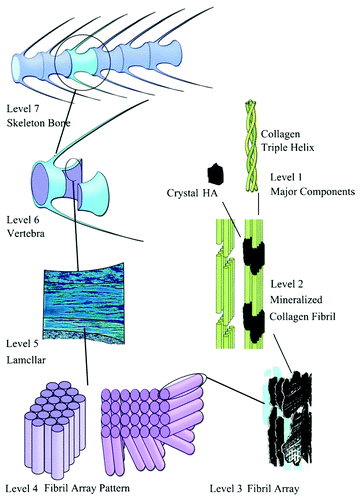
Figure 12. A schematic illustration of in vivo mineralization of a collagen fibril: top layer-calcium orthophosphate clusters (green) form complexes with biopolymers (orange line), forming stable mineral droplets; second top layer-mineral droplets bind to a distinct region on the collagen fibers and enter the fibril; second bottom layer-once inside the collagen, the mineral in a liquid state diffuses through the interior of the fibril and solidifies into a disordered phase of ACP (black); bottom layer-finally, directed by the collagen, ACP is transformed into oriented crystals of biological apatite (yellow). Reprinted from reference Citation628 with permission.
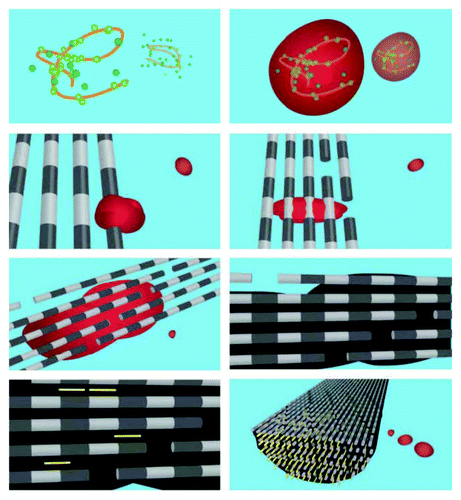
Figure 13. A schematic drawing of a tooth. Other very good graphical sketches of the mammalian tooth structure, including the hierarchical levels, are available in references Citation509 and Citation554.
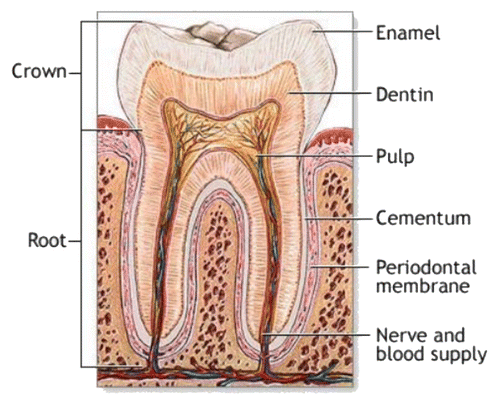
Figure 15. Red deer stag at velvet shedding. The bare bone of the hard antlers is exposed. Reprinted from reference Citation731 with permission. A good cross-sectional image of a deer antler is available in reference Citation554.

Table 4. Occurrence of calcium phosphates in biological systems (human)Citation84
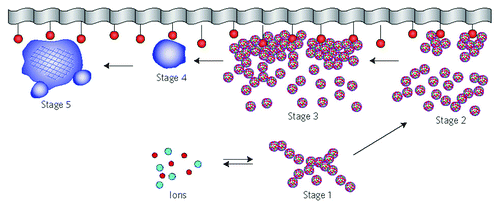
Figure 16. A schematic representation of the different stages of a surface-directed mineralization of calcium orthophosphates. In stage 1, aggregates of pre-nucleation clusters are in equilibrium with ions in solution. The clusters approach a surface with chemical functionality. In stage 2, pre-nucleation clusters aggregate near the surface, with loose aggregates still in solution. In stage 3, further aggregation causes densification near the surface. In stage 4, nucleation of spherical particles of ACP occurs at the surface only. In stage 5, crystallization occurs in the region of the ACP particles directed by the surface. Reprinted from references Citation628 and Citation828 with permission.