Figures & data
Figure 1. Three-dimensional structure of human IL-8 in solution as determined by NMR spectroscopy. The figure was constructed using the coordinates deposited in the Protein Data Bank (accession code pdb1IL8) by the pymol software (version 0.99). Due to the high concentrations used in structural studies IL-8 is present as a dimer, where the two α-helices are arranged in antiparallel fashion on top of a six stranded β-sheet. The receptor binding site, including the N-terminal receptor binding motif ELR (Glu-Leu-Arg) and the loop region from residue 30–36, is shown in yellow. GAG-binding regions, shown in red, comprise the residues 18–23 in the proximal loop and the α-helical domain. Of note, receptor- and GAG-binding sites are spatially separated. Further details are given in the text.
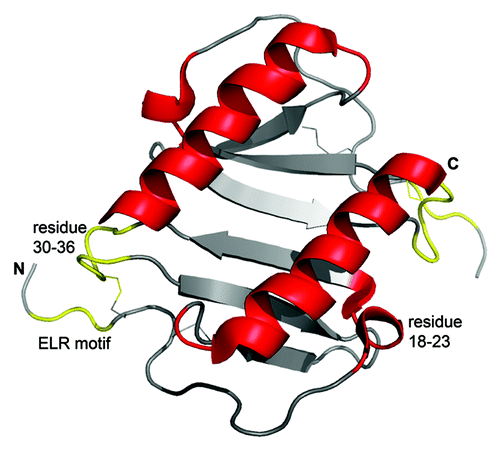
Table 1. Glycosaminoglycans of the extracellular matrix
Table 2. Important proteoglycans of cell surfaces and extracellular matrices
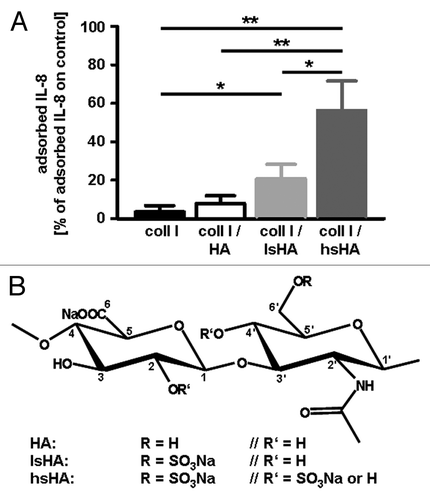
Figure 2. (A) Adsorption of human macrophage-derived IL-8 to modified hyaluronan containing different levels of sulfation. For adsorption of IL-8, native and differentially sulfated hyaluronan (kindly provided by Dr Moeller, INNOVENT e.V. Jena, Germany) immobilized on a collagen matrix were incubated with supernatants of LPS stimulated human monocyte-derived macrophages containing 138 ± 46 ng/ml IL-8. Tissue culture polystyrene was used as control substrate. After 24 h at 37°C, the supernatants were harvested and amounts of IL-8 determined by ELISA. Level of adsorbed IL-8 was calculated from the difference of the IL-8 amount determined in supernatants from the control substrate and of the IL-8 amount determined in supernatants from the different hyaluronan matrices, respectively. All data are mean (± SD) of three independent experiments. *p < 0.05; **p < 0.005 (t-test); (B) Chemical structures of native HA and sulfated HA derivatives.Citation59 coll I, collagen I; HA, hyaluronan; lsHA, low sulfated HA; hsHA, high sulfated HA