Figures & data
Figure 1.vegf-a. (A) Gene structure. TSS is the transcriptional start site. (B) mRNA species. Alternative splicing of the vegf-a gene in the terminal exon results in two families of isoforms: the pro-angiogenic VEGFxxx and the anti-angiogenic VEGFxxxb isoforms. AUG, the start site for translation; UTR, the untranslated region; pA, the polyadenylation site. (C) Protein structure of the two major isoforms of each family, VEGF165 and VEGF165b. C-terminal splicing leads to an alternative last six amino acids (CDKPRR or SLTRKD). The isoforms are termed according to the amino acid number of the resulting protein (xxx). HSPG, heparin sulfate proteoglycan; R1, VEGF Receptor 1; R2, VEGF Receptor 2 (from ref. Citation29).
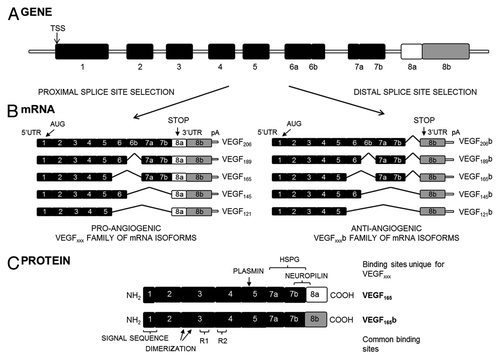
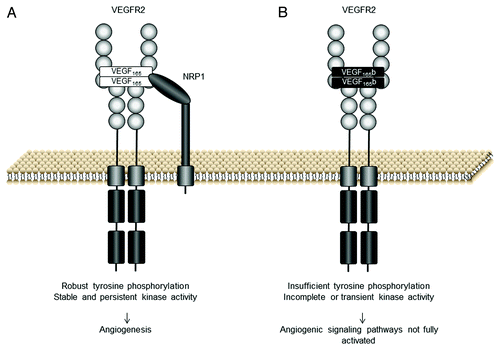
Figure 2. VEGF165b and VEGF165 interaction with VEGF receptor 2 (VEGFR2). (A) The VEGFR2 binding site of VEGF165 interacts with the VEGFR2 extracellular domain. VEGF165 functions as a dimer and promotes the formation of VEGFR2 dimers resulting in activation of the kinase domains via the phosphorylation of its tyrosine residues. Robust tyrosine phosphorylation results in the activation of angiogenic signaling pathways. (B) VEGF165b binds the VEGFR2 binding site with equal affinity to VEGF165 but does not bind neuropilin 1 (NRP1) co-receptor. The C-terminus of VEGF165b is neutral and there is insufficient torsional rotation for complete tyrosine phosphorylation, although weak phosphorylation can occur (adapted from ref. Citation11)