Figures & data
Figure 1 Nutlin induced selective growth inhibition and activated the p53 pathway in MM cells harboring wt p53. (A) Nutlin suppressed the growth of MM cells harboring wt p53. MM cells harboring either wt, mt or null p53 were treated with different dose of nutlin. After 48 hrs, effects of nutlin on the viability of MM cells were assessed using MTT cell viability assay. The survivals of nutlin-treated cells are expressed as percent of the DMSO-treated control. Results are expressed as mean ± SD. (B) Time-dependent activation of the p53 signaling in response to nutlin treatment. Whole cell lysates were prepared at the indicated hours after treatment of MM1.S and H929 cells with 5 µM nutlin and analyzed by WB for the indicated proteins. (C) Dose response of nutlin-induced activation of the p53 pathway in MM cells. At 24 hrs after treatment with 2.5–10 µM nutlin, whole cell lysates from MM cells harboring either wt (MM1.S or H929) or mt (LP1) or null p53 (8226R5) were analysed for the expression of p53 and its two immediate downstream targets, p21 and MDM2 by WB analysis. β-actin was used as loading controls.
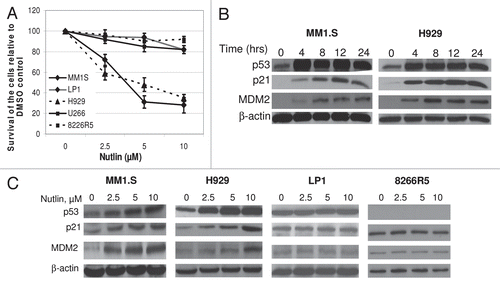
Figure 2 Inhibition of the p53-MDM2 interaction by nutlin induced p53-dependent apoptosis. Annexin-V binding and PI uptake in cells harboring either wt or mt p53 were examined as a marker for induction of apoptosis after treatment of the cells with nutlin. (A) MM1.S cells were incubated with 2.5–10 µM nutlin for different time periods (24–72 hrs) to determine the time and dose responses for nutlin-induced apoptosis in this cell. (B) FCM profiles of MM1.S, H929 and LP1 cells treated for 48 hrs with 5 µM nutlin or DMSO control. Results of a single representative experiment from a set of at least three experiments are shown. (C) Percent specific apoptosis were calculated as described in material and methods and shown in the figure. Results showed significant amount of apoptosis in cells harboring wt p53 but not in cells expressing mt or null p53. Results are expressed as mean ± SD.
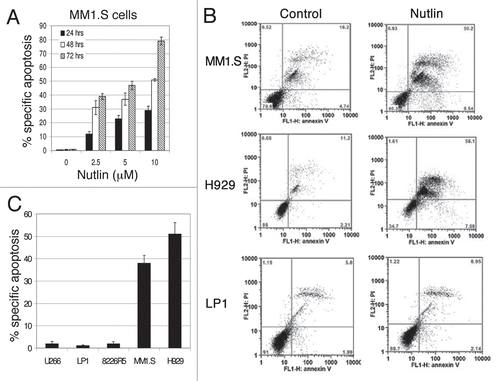
Figure 3 Nutlin-induced apoptosis was mediated through regulation of apoptotic targets. (A) MM cells were treated with 5 µM nutlin for 24 hrs. Whole cell lysates from the indicated cells were analysed for expression of the indicated proteins by WB analysis. Nutlin-induced upregulation of the p53 along with a pro-apoptotic target, PUMA and downregulation of an anti-apoptotic protein, Bcl2 was observed in wt p53 harboring MM1.S and H929 cells. By contrast, no significant changes in the levels of these proteins were detected in mt p53 expressing U266 or p53 null 8226R5 cells. Apoptosis in these cells was further confirmed by cleavage of caspase-3. (B) Nutlin-induced apoptosis is mediated by both extrinsic and intrinsic pathways in MM cells. H929 cells were treated with different doses of nutlin for 24 hrs and activation of caspase together with pro- and anti-apoptotic targets were examined by WB analysis. Nutlin induced activation of both caspase-8 and caspase-9 followed by induction of caspase-3. The activation of caspase in H929 cells was associated with induction of pro-apoptotic proteins, PUMA, Bax and Bak and repression of anti-apoptotic proteins, Bcl2 and survivin. (C) Nutlin-induced apoptosis in MM cells was caspase-dependent. MM1.S and H929 cells were treated with 10 µM pan-caspase inhibitor for 1 hr before treating the cells with 5 µM nutlin for 24 hrs. Activation of caspase and PA RP was examined by WB analysis. Inhibition of capsase-activation by pan-caspase inhibitor, ZVAD-FMK resulted in blocking of caspase activation and thus inhibition of apoptosis.
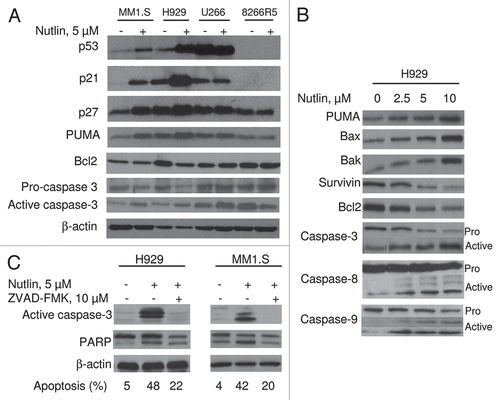
Figure 4 Nutlin inhibited the growth of primary MM samples and stabilized p53. (A) Primary MM samples were treated with different dose of nutlin for 48 hrs and survival of the cells was measured by MTT cell survival assay. Nutlin induced dose-dependent inhibition of the proliferation of cells in 3 of the 5 primary samples. The survivals of nutlin-treated cells are expressed as percent of the DMSO-treated control. Results are expressed as mean ± SD. (B) Primary MM samples were treated with different dose of nutlin for 24 hrs and activation of the p53 pathway was examined by induction of p53 and its downstream targets. Nutlin induced p53 activation in two representative samples in (A), however, the induction of p53 was not observed in the sample #3, in which inhibition of proliferation was not observed by MTT assay.
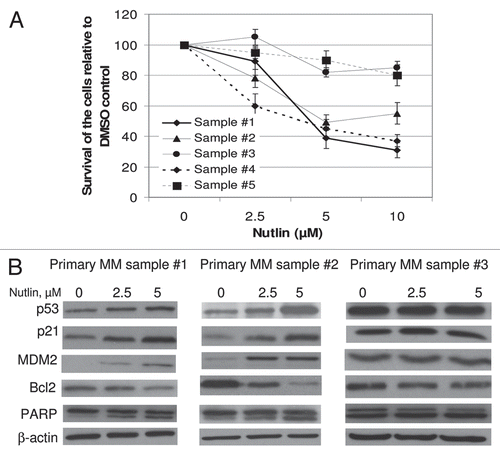
Figure 5 Transcriptional blockade by PFT-α induced apoptosis of MM1.S cells. Cells were pre-treated with 15 µM PFT-α for 4 hrs followed by 5 µM nutlin for additional 6 hrs. (A) Immunoblot indicating that pretreatment of MM1.S cells with PFT-α repressed p53 transcriptional targets p21, MDM2, PUMA and Bax upon nutlin treatment, while not altering p53 levels. (B) Immunoblot showing expression of p53 and its transcriptional targets in nutlin-induced PFT-α treated LP1 cells. (C) Quantitation of apoptosis assay by FCM for nutlin-induced MM1.S and LP1 cells pretreated with PFT-α. Pretreatment of MM1.S cells with PFT-α augmented the apoptotic response to nutlin. PFT-α did not interfere with nutlin-induced apoptosis in LP1 cells. Data are mean ± SD of duplicate measurements. (D) Gene expression analysis by qRT-PC R in nutlin-induced MM1.S and LP1 cells in the presence or absence of p53 transcriptional inhibitor, PFT-α.
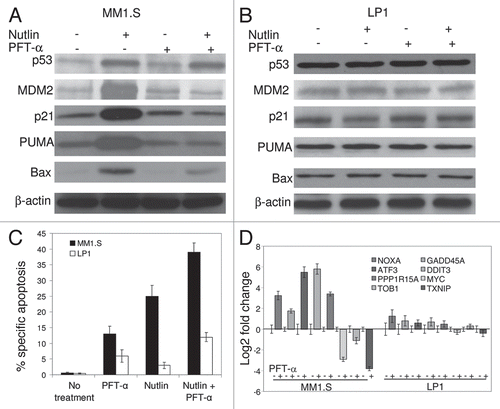
Figure 6 Nutlin-induced apoptosis was associated with translocation of p53 to mitochondria. (A) Nutlin-treated MM1.S cells were stained for p53 (green) and mitochondrial marker protein COXIV (red) and visualized by confocal microscopy. Localization of p53 to mitochondria is indicated by the yellow-orange color in the merged images. A preferential translocation of cytoplasmic p53 to mitochondria suggests that cytoplasmic p53 mediates apoptosis mainly at the level of mitochondria. (B) Nutlin-induced MM cells were fractionated to yield nuclear, mitochondrial and cytosolic fractions. Accumulation of p53 in mitochondrial and cytosolic fractions in addition to nuclear fraction was assessed by western blotting. Nutlin stabilized wt p53 in both nucleus and cytoplasm and promoted translocation of p53 to mitochondria followed by cytochrome (cyt. C) release. The purity of the subcellular fractions was verified by using H2A, COXIV and β-tubulin for detecting nuclear-, mitochondrial- and cytosol-specific proteins, respectively.
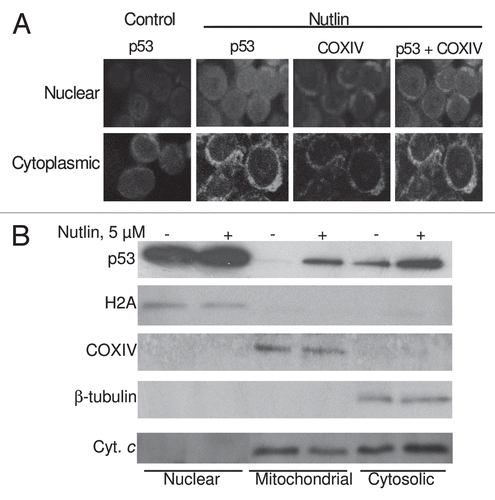
Figure 7 Role of the p53-Bcl2 interaction in nutlin-induced apoptosis. (A) Nutlin-induced p53 binding to mitochondrial Bcl2 protein. MM1.S and H929 cells were treated with 5 µM nutlin or DMSO control for 6 hrs. After which CHAPS lysates together with MM1.S whole cell lysates (w.c.l.) were prepared and analyzed directly or (B) following immunoprecipitation with anti-Bcl2 antibody. Nutlin-induced upregulation of p53 was shown in MM1.S whole cell lysates as well as CHAPS lysates of both MM1.S and H929 cells. Similar to this observation, p53 protein expression precipitated by Bcl2 in the cytosolic extracts of MM1.S and H929 cells was also increased after treatment with nutlin. (C) Immunoprecipitation supernatants were analyzed for COXIV to document equal inputs.
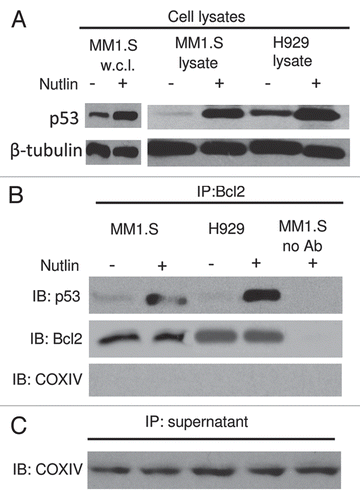
Figure 8 Blocking of mitochondrial translocation of p53 did not inhibit apoptosis induction by nutlin. (A) MM1.S cells pretreated with PFT-µ, a specific inhibitor of mitochondrial translocation of p53, were treated with 5 µM nutlin for 6 hrs. Accumulation of p53 was studied by mitochondrial fractionation of the cells followed by WB analysis. Treatment of cells with PFT-µ resulted in inhibition of mitochondrial translocation of p53. β-tubulin and COXIV served as loading controls as well as cytosolic and mitochondrial markers, respectively. However, PFT-µ did not prevent either mitochondrial cytochrome (cyt. c) release or cleavage of PA RP in nutlin-induced cells. (B) PFT-µ did not inhibit apoptosis induction by nutlin in MM cells. MM1.S cells pretreated with PFT-µ were treated with 5 µM nutlin and apoptosis was measured by annexin-V staining at two different time periods. Consistent with the results of cytochorme C release and PARP cleavage shown in (A), pretreatment of MM1.S cells with PFT-µ did not inhibit the apoptotic response to nutlin.
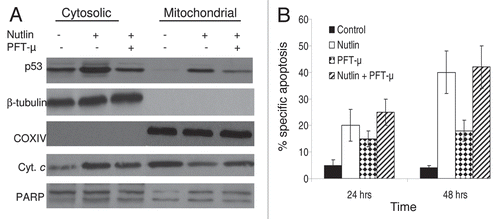
Table 1 Differentially expressed genes in nutlin-induced PFT-α treated MM1.S cells