Figures & data
Figure 1 Deciphering tumor-stroma co-evolution: the autophagic tumor stroma model of cancer cell metabolism. Cancer cells induce oxidative stress in adjacent stromal fibroblasts. Then, the resulting oxidative stress in cancer-associated fibroblasts helps drive tumor-stroma co-evolution, by randomly mutagenizing cancer cells, while protecting them against apoptosis and providing them with abundant recycled nutrients and chemical building blocks via autophagy. This results in a net energy transfer from the autophagic tumor stroma to the anabolic “hungry” cancer cells. Thus, stromal oxidative stress and autophagy function(s) as a “battery” to drive tumor-stroma co-evolution and “fuel” oxidative mitochondrial metabolism in cancer cells. A+ (positive), increased autophagy/mitophagy in cancer associated fibroblasts; A− (negative), decreased autophagy/mitophagy in epithelial cancer cells. AR (resistant), denotes the successful evolution of autophagy-resistant cancer cells, due to genetic silencing or deletion of required autophagy genes, such as Beclin1.
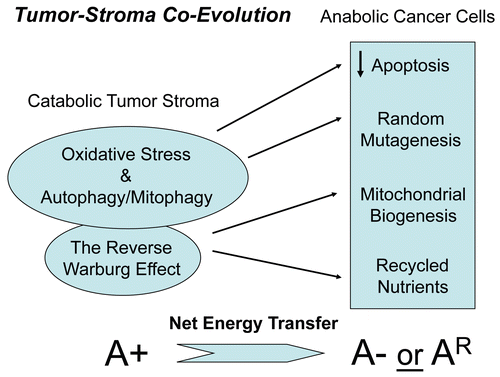
Figure 2 Acute knock-down of Cav-1 in human stromal fibroblasts increases ros production and negatively affects mitochondrial activity. (Upper) Cav-1 knock-down induces ROS production. CM-H2DCFDA staining (green) was performed on hTER T-fibroblasts treated with Cav-1 siRNA (right) or control siRNA (left). Cells were counterstained with Hoechst nuclear stain (blue). Samples were then immediately imaged using a 488 nm excitation wavelength. Note that Cav-1 knock-down greatly promotes ROS generation. Importantly, images were acquired using identical exposure settings. Original magnification, 20x. (Lower) Cav-1 knock-down decreases mitochondrial activity. hTERT-fibroblasts were treated with Cav-1 siRNA (right) or control siRNA (left). Then, functional mitochondria with active membrane potential were visualized using MitoTracker staining (red). DAPI was used to stain nuclei (blue). Note that transient Cav-1 knock-down greatly decreases mitochondrial activity. Importantly, paired images were acquired using identical exposure settings. Original magnification, 63x. Images were reproduced from references,Citation7 and Citation7 with permission.
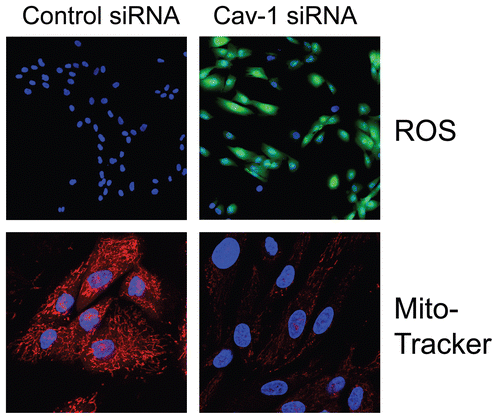
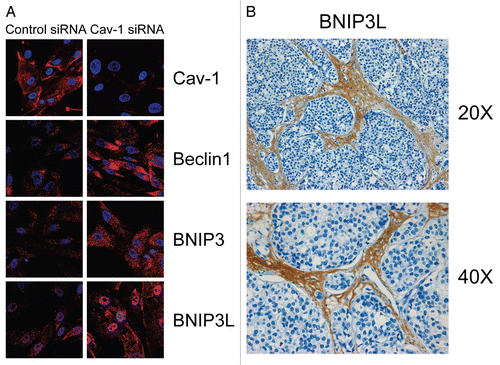
Figure 3 Acute knock-down of Cav-1 in human stromal fibroblasts activates autophagy and mitophagy: implications for human breast cancer. (A) Acute loss of Cav-1 increases the expression of autophagic markers. hTER T-fibroblasts were treated with Cav-1 siRNA or control (CTR) siRNA. Cells were fixed and immuno-stained with antibodies against Beclin1, BNIP3 and BNIP3L. DAPI was used to visualize nuclei (blue). Importantly, paired images were acquired using identical exposure settings. Original magnification, 40x. Note that acute Cav-1 knockdown is sufficient to greatly increase the expression levels of all the autophagy/mitophagy markers we examined. (B) BNIP3L is highly increased in the stroma of human breast cancers that lack stromal Cav-1. Paraffin-embedded sections of human breast cancer samples lacking stromal Cav-1 were immuno-stained with antibodies directed against BNIP3L. Slides were then counter-stained with hematoxylin. Note that BNIP3L is highly expressed in the stromal compartment of human breast cancers that lack stromal Cav-1. Original magnification, 20x and 40x, as indicated. Images were reproduced from the references Citation7 and Citation8, with permission.