Figures & data
Figure 1. Sin3B stabilizes exogenously expressed Mad4. (A) SF767 cells were transfected with FLAG-Mad4, FLAG-mSin3B and empty vector (EV) such that the total amount of plasmid used in each treatment was equal. After 42 to 48 h, cells were lysed in sample buffer and protein expression was measured by western blot analysis using the indicated antibodies. (B) U251 or U373 cells were transfected with the indicated plasmids and lysed in sample buffer after 42–48 h. Protein expression was determined by immunoblotting. (C) U251, U373 and SF767 cells were transfected with 8 µg Sin3B or empty vector in addition to 1 µg GFP expression vector. After 72 h, protein expression was assessed by immunoblotting as indicated.
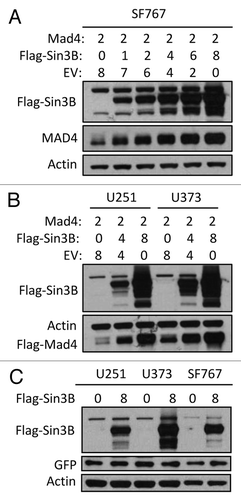
Figure 2. Sin3B stabilizes endogenous Mad4 protein. (A) The same samples used in were assessed for endogenous Mad4 expression. (B) SF767 cells were transfected with 4 µg of empty plasmid vector or plasmids carrying human Sin3A and Sin3B. After 48 h, cells were assessed by immunoblotting as indicated. (C) H460 cells were radiated with 5 Gy and harvested at the times indicated. The expression levels of Sin3B and Mad4 over time were examined by immunoblotting. (D) Expression of Sin3B and Mad4 expression after irradiation of A172 cells with 5 Gy (C, control). (E) SF767 or U373 cells in 60-mm dishes were irradiated twice with 5 Gy each treatment over 2 d and subsequently harvested every 24 h. The expression levels of different proteins were measured as indicated (C, control). (F) Expression of Sin3B and Mad4 expression in A172 cells treated with temozolomide at increasing concentration for 43 h.
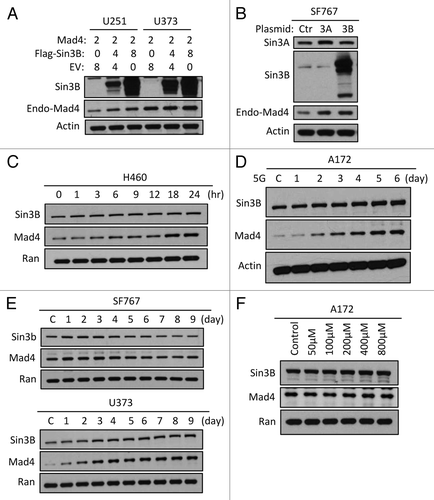
Figure 3. Sin3B, Sin3A and c-Myc influence Mad4 stability. (A) Sin3A or Sin3B expression was silenced in four GBM cell lines by siRNA and c-Myc, Mad4 and β-actin expression was determined by immunoblotting. (B) SF767 cells were transfected with siRNA to knock down c-Myc expression. After 3 d, protein expression was assessed by immunoblotting. (C) Cells were transfected with siRNA oligos (30 pmol/well in 24-well plates) to knock down expression of Sin3B. After 2 d, cells were transfected with Flag-Mad4 plasmids at 2 μg per well. After 24 h, cells were assessed by immunoblotting. (D) Upper panel: SF767, U251 or U373 cells were transfected with 4 μg of Flag-Sin3A or c-Myc plasmid and 2 μg of Flag-Mad4 plasmid. After 48 h, the cell lysates were assessed by immunoblotting. Lower panel: Cells from four cell lines were lysed and assessed by immunoblotting as indicated. (E) SF676 cells were transfected with siRNA to knock down Sin3A and Sin3B expression and protein expression was assessed as indicated. (F) Left, expression of Sin3B in Sin3B wild-type or knockout MEFs; right, Sin3B wild-type or knockout MEFs were transfected with siRNA to deplete Sin3A expression and Mad4 expression was measured by immunoblotting. (G) U373 cells were transfected with siRNA to knockdown expression of Sin3A and Sin3B (AB) or Sin3A, Sin3B and c-Myc (ABM) (20 pmol each in 24-well plates) and protein expression level was determined as indicated. (H) TGR1 (c-Myc+/+) or HO15.19 (c-Myc−/−) cells were transfected with siRNA to silence expression of both Sin3A and Sin3B and the expression of Mad4 protein was assessed by immunoblotting.
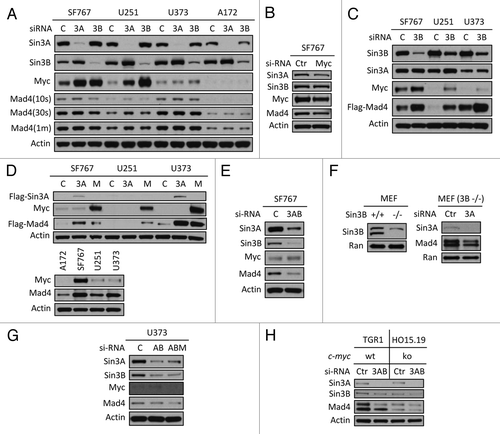
Figure 4. Mad4 is targeted by c-IAP1 for degradation. (A) GBM cell lines were transfected with 6 µg of Flag-tagged c-IAP1 expression vector (Flg-P1) or control vector. After approximately 40 h, the expression level of endogenous Mad4 was determined by immunoblotting. (B) A172 cells were transfected with 8 µg of Flag-cIAP1 or H588A mutant or 12 µg of RING domain deletion mutant. After approximately 2 d, the expression levels of Sin3A, Sin3B, c-Myc and Mad4 were determined by immunoblotting (1 min, 1 min exposure; O/N, film exposed overnight). (C) U373 cells were transfected with 5 µg of Flag-tagged c-IAP1 or c-IAP1-H588A or 10 µg of RING domain deletion mutant and after 40–46 h cell lysates were analyzed by immunoblotting as indicated. (D) U251 cells were transfected with 6 µg of Flag-tagged c-IAP1 or c-IAP1-H588A or 10 µg of RING domain deletion mutant and after 40–46 h cell lysates were analyzed by immunoblotting as indicated. (E) SF767 cells were transfected with 6 µg of Flag-tagged c-IAP1 or c-IAP1-H588A or 12 µg of RING domain deletion mutant and after 40–46 h cell lysates were analyzed by immunoblotting as indicated.
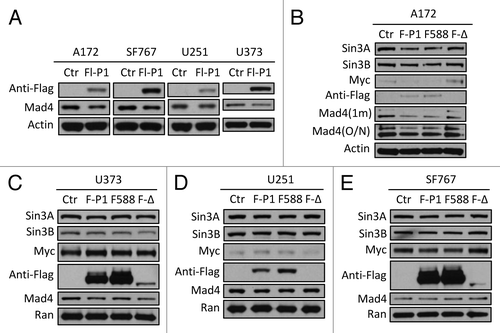
Figure 5. Sin3B interferes with c-IAP1-mediated degradation of Mad4. (A) The plasmid Flag-Sin3B expressing Sin3B of human origin was transfected into four GBM cell lines and after 2 d cell lysates were analyzed for c-IAP1 expression by immunoblotting. (B) Knockdown of Sin3B in four cell lines was performed and c-IAP1 levels were examined by immunoblotting. (C) U251 cells were transfected with anti-human Sin3B siRNA and 2 d later the same cells were transfected with Flag-c-IAP1. After 40–44 h, cells were assessed by immunoblotting. (D) Cells from four cell lines were lysed in immunoprecipitation (IP) buffer and the lysates were pre-cleared by rabbit-IgG agarose and immunoprecipitated by rabbit anti-human c-IAP1 antibody. Immune complexes were captured by Protein G agarose. The co-precipitated proteins were assessed by immunoblotting as indicated (SE/LE, short/long exposure). (E) Cell lysates from SF767 or U373 cells in IP buffer were immunoprecipitated with goat anti-human Mad4 polyclonal antibody and analyzed with immunoblotting as indicated. Mad4 was analyzed with goat anti-human Mad4 antibody for input and with rabbit anti-human Mad4 antibody for IP. (F) U373 cells were transfected with Flag-Sin3B plasmids and lysed in IP buffer after 2 d. IP was performed using rabbit anti-human c-IAP1 polyclonal antibody and co-immunoprecipitated proteins were assessed as indicated. (G) U251 or U373 cells were transfected with Flag-Sin3B plasmids and treated with IP buffer after 2 d. Co-immunoprecipitated proteins were assessed by immunoblotting. (H) SF767/U373 cells were transfected with mouse Flag-Sin3B and U373/U251 cells were transfected with human Flag-Sin3B plasmids. After 2 d, protein expression was assessed by immunoblotting as indicated.
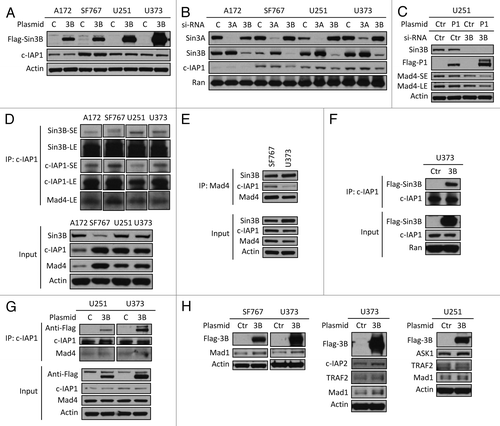
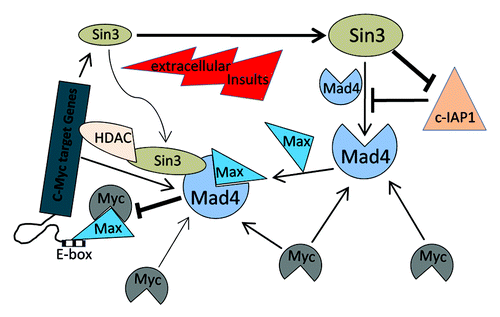
Figure 6. Schematic representation of the regulation of Mad4 in GBM cells by Sin3 and c-Myc. Sin3B (as well as Sin3A) maintains the steady-state level of Mad4 partly by counteracting c-IAP1-mediated degradation of Mad4. Mad4 competitively sequesters c-Myc from its binding partner Max, thus suppressing c-Myc transactivities. Furthermore, the unbound c-Myc molecules may facilitate Mad4 protein accumulation. Upon extracellular insults, such as gamma radiation, Sin3B and Mad4 are increased, which may compromise c-Myc stability and thus interfere with c-Myc-mediated cell proliferation or further silence c-Myc targeted genes through Sin3B-linked HDAC activity. (→, enhancement; ⊣, inhibition).