Figures & data
Figure 1. Baseline expression of p16/CDK4/Rb signaling molecules in 5 cancer cell lines. Constitutive basal expression of Rb, p-Rb, CDK4, CDK6, and p16INK4A were detected by western blotting. Actin was used as a loading control.
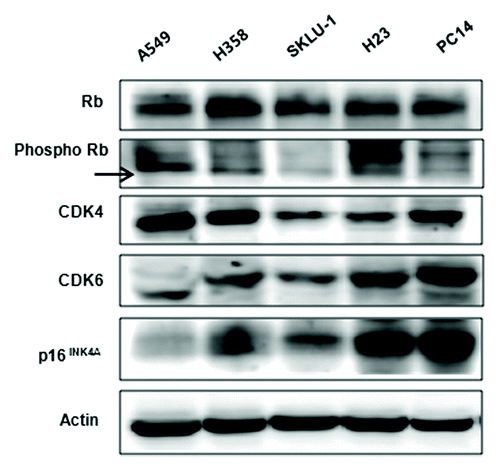
Figure 2. Efficient inhibition of CDK4 mRNA and protein and changes in cell cycle distribution in H23 cells transfected with CDK4 siRNA. (A) Expression of CDK4 mRNA examined by RT-PCR 48 h after CDK4 siRNA transfection. GAPDH was used as a loading control. (B) Expression of CDK4 and pRb protein was significantly reduced 48 h after CDK4 siRNA transfection. Actin was used as a loading control. (C) Cell cycle distribution. G0/G1 phase increased 48 h after CDK4 siRNA transfection of H23 cells. Each experiment was performed in triplicate. Data represent the mean ± SD. Statistically significant differences between the CDK4 siRNA and control are presented as *(P < 0.05).
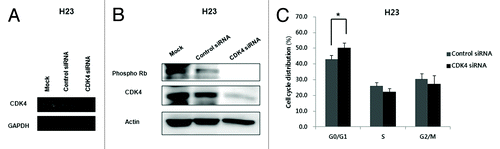
Figure 3. Enhanced anti-proliferative effect of paclitaxel in CDK4 siRNA-transfected H23 cells. When exposed to the different concentrations of paclitaxel (1, 3, 5, and 10 nM), a concentration-dependent increase of anti-proliferative effects was observed in the CDK4 siRNA-transfected cells. Each experiment was performed in triplicate. Data represent the mean ± SD. Statistically significant differences between the CDK4 siRNA and control are presented as * (P < 0.05) and ** (P < 0.01).
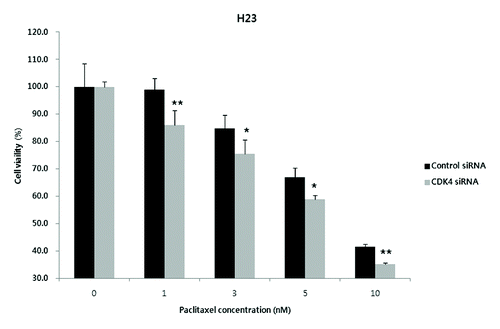
Figure 4. Anti-proliferative effect of a CDK4/6 inhibitor, CINK4, in 5 lung adenocarcinoma cell lines. When treated with various concentrations of CINK4 (0.1–40 μM), the anti-proliferative effects of CINK4 were similar for each cell line at 72 h. Cell viability was quantitatively measured by SRB assay and presented as a percentage of the control cell population. Each experiment was performed in triplicate. Data represent the mean ± SD.
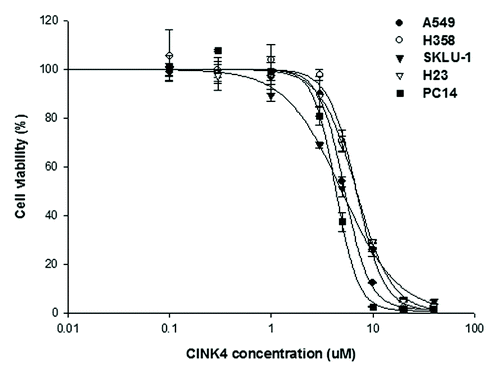
Table 1. Sensitivity of NSCLC cell lines to CINK4
Figure 5. The effects of CINK4 on cell cycle distribution and apoptosis in KRAS mutation-bearing A549 and H23 cells. (A) Changes in cell cycle distribution were measured by flow cytometry after CINK4 treatment for 48 h. Each experiment was performed in triplicate. Data represent the mean ± SD. Statistically significant differences between CINK4 treatment groups and the control were presented as *(P < 0.05) and ** (P < 0.01). (B) Apoptotic cells induced by CINK treatment (5 and 10 μM) were measured by flow cytometry. Annexin V-FITC/PI double staining was performed and Annexin V-positive apoptotic cells were counted by flow cytometry. Each experiment was performed in triplicate. Data represent the mean ± SD. Statistically significant differences between CINK4 treatment groups and controls are presented as ** (P < 0.01) and *** (P < 0.001). (C) The effects of CINK4 on cell cycle regulators and apoptosis-related molecules were evaluated by western blotting. A549 and H23 cells were treated with CINK4 (3, 5, and 10 µM) for 72 h. Actin was used as a loading control.
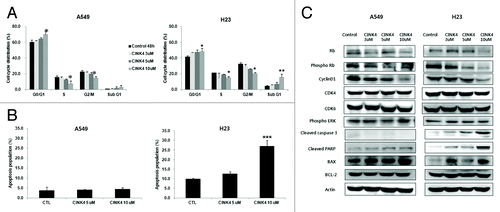
Figure 6. Anti-proliferative effects of paclitaxel alone and combined with CINK4 in 3 KRAS mutation-positive NSCLC cell lines. (A) Anti-proliferative effect of paclitaxel (0.1–300 nM) in 3 KRAS mutation-positive NSCLC cell lines at 72 h. (B) Paclitaxel (1, 3, 5, and 10 nM) was combined with CINK4 (1, 3, 5, and 10 μM) for 72 h in individual cell line. Interactions between paclitaxel and CINK4 were analyzed by combination index (CI). The calculated CIs were divided into 3 categories: CI < 1, CI = 1, or CI > 1, indicating synergistic, additive and antagonistic effects, respectively.
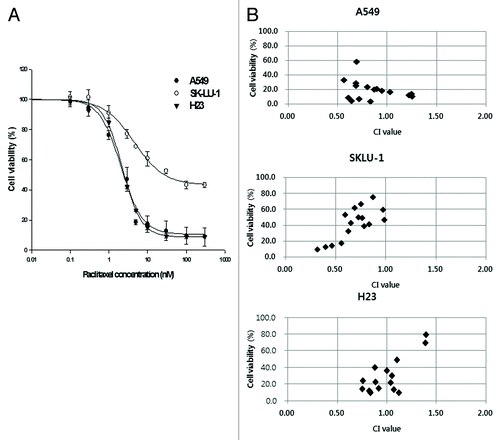
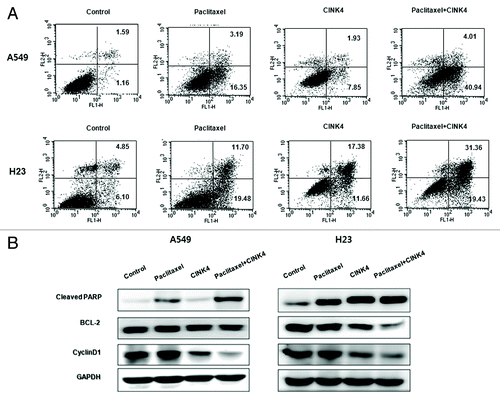
Figure 7. Paclitaxel combined with CINK4 increases apoptosis in A549 and H23 cells. (A) Apoptosis induction. Annexin V-FITC/PI double staining was performed. Annexin V-positive apoptotic cells were counted by flow cytometry. Each experiment was performed in triplicate. The typical apoptosis induction by single and combination treatments are shown. (B) Apoptosis-related protein expression. Actin was used as a loading control. Cells were treated for 72 h with paclitaxel (3 nM) and CINK4 (10 μM), alone or in combination.