Figures & data
Figure 1. Neural stem-like cells are present in GBM xenografts. (A) Representative pictures of a primary and a sub sphere culture. Scale bar shows 100 µM. (B) Sub sphere formation in later cultures. Bar chart represents the mean number of sub spheres formed per 100 cells plated ± SEM (C) Representative sphere formed from a single cell. (D) Representative pictures of differentiated sphere cells expressing GFAP (astrocytic marker), CNPase (oligodendrocytic marker), or βIII-tubulin (neuronal marker) upon serum addition. Expression was detected by immunocytochemical staining. WB analysis of the NSC marker Nestin in (E) subcutaneous GBM xenografts and (F) thereof derived neurosphere cultures.
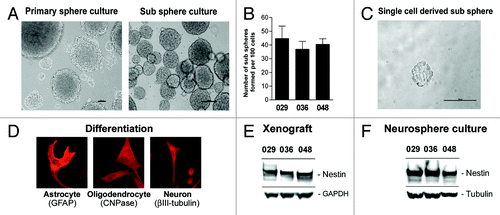
Figure 2. Expression of Notch-1 and the Notch target Hes-1 is maintained from xenograft to culture. Basal protein expression of the Notch-1 receptor and the Notch transcriptional target Hes-1 in (A) subcutaneous xenograft tumors from which the neurosphere cultures used in this study were derived and in (B) the xenograft-derived GBM neurosphere cultures.
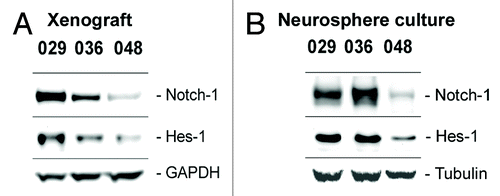
Figure 3. Notch inhibition more profoundly affects cultures with high Notch expression and activation. (A) Q-RT-PCR analysis of Hes-1 mRNA expression. Bars represent mean normalized Hes-1 mRNA expression ± SEM in control and DAPT treated cultures from two independent experiments performed in duplicates. Comparisons of means between control and treated were based on the unpaired t-test performed on the normalized data. Stars represent the difference between the mean of control and treated. **P < 0.01. (B) WB detection of Hes-1 protein in DAPT and DMSO treated cultures. In (A and B) the cells were treated with 5 µM DAPT or DMSO for two weeks. (C) Cell cycle analysis of neurosphere cultures treated with 5 µM DAPT or DMSO for three days. Bars represent the difference in the G0/G1 fraction ± SEM between the DMSO control and the DAPT treated samples from three independent experiments. Positive bars correspond to an increase in the G0/G1 fraction in the DAPT treated samples compared with the control. Comparisons of means between control and treatment were based on the paired t-test performed on the untransformed data. Stars represent the difference between the mean of control and treated. **P < 0.01, ***P < 0.001. (D) WB detection of Hes-1, cell cycle regulators (CDK4 and p21), and apoptotic markers (cleaved caspase-3 and BAX) in neurosphere cells treated with 5 µM DAPT or DMSO for 3 d.
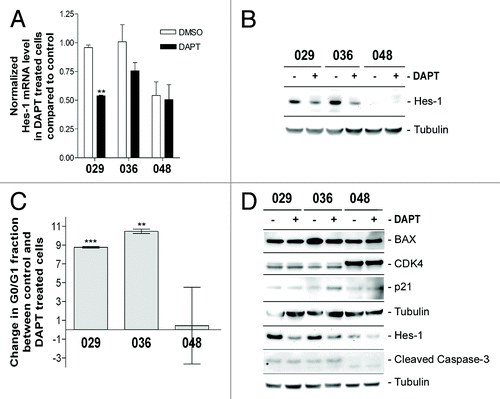
Figure 4. Notch inhibition hampers in vitro tumorigenic potential. Colony formation assay in soft-agar with the addition of 10 µM DAPT or DMSO performed on (A) DAPT-naïve cells or (B) DAPT-pretreated cells. Bars shows the relative mean of colonies formed after 14 d ± SEM. Stars represent the difference between the mean of control and treated. *P < 0.05, ***P < 0.001. (C) Representative photos of the colony formation assay.
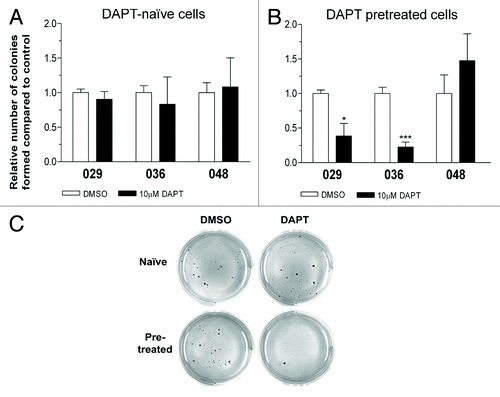
Figure 5. DAPT treatment affects primary neurosphere formation but not sub sphere formation or differentiation level. (A) Primary sphere formation assay performed on acutely dissociated GBM xenograft cells with the addition of 0, 1, 5, or 10 µM DAPT. Notice that 0µM DAPT was used as control for 029p5, 036p8, 047p2, and 048p7, while DMSO was used for 017p4 and 036p15. (B) WB detection of Notch-1 in xenograft tumor tissue from which the acutely dissociated GBM cells for the primary sphere assay were derived. (C) Sub-sphere formation assay performed on naïve cells treated with 5 µM DAPT or DMSO or (D) pretreated cells with the addition of 10µM DAPT or DMSO. In (A, C, and D) bars show relative mean of spheres formed after 14 d ± SEM. Stars represent the difference between the mean of control and treated. *P < 0.05, **P < 0.01, ***P < 0.001. N.A., not analyzed. (E and F) WB detection of markers for differentiation in neurosphere cells treated in parallel to (C and D) respectively. GFAP for astrocytes, βIII-tubulin for neurons and CNPase for Oligodendrocytes. Naïve cells are displayed in (E) and pretreated in (F).
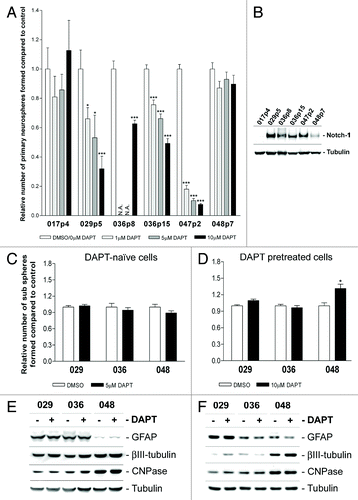
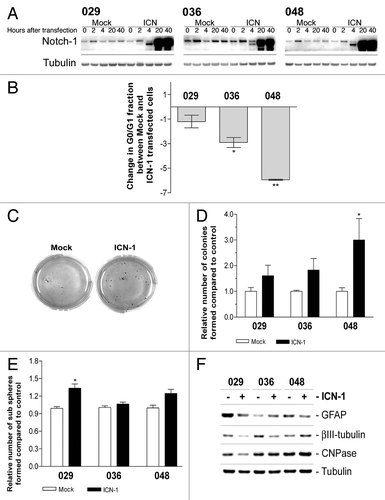
Figure 6. Transfection with ICN-1 results in opposite effect from DAPT treatment and affects stem cell characteristics. (A) Expression of total Notch-1 in cultures transfected with ICN-1 or mock plasmid after 0, 2, 4, 20, or 40 h. (B) Cell cycle analysis of neurosphere cultures transfected with ICN-1 or Mock and left for three days. Bars represent the difference in the G0/G1 fraction ± SEM between Mock and ICN-1 transfected samples from at least two independent experiments performed in mono- or duplicates. Negative bars correspond to a decrease in the G0/G1 fraction in the ICN-1 transfected samples compared with the mock samples. Comparisons of means between Mock and ICN-1 were based in the paired t-test performed on the untransformed data. (C) Representative photos of soft-agar assay performed on cells transfected with ICN-1 or Mock. (D) Quantification of colonies formed in the soft-agar assay with ICN-1 or Mock transfection. Bars shows the relative mean of colonies formed after 14 d ± SEM (E) Sub sphere formation assay performed on cells transfected with ICN-1 or Mock. Bars show relative mean of spheres formed after 14 d ± SEM. For (B, D, and E) stars represent the difference between the mean of control and treated. *P < 0.05, **P < 0.01. (F) WB detection of markers for differentiation in neurosphere cells treated parallel to (E). GFAP for astrocytes, βIII-tubulin for neurons, and CNPase for oligodendrocytes.