Figures & data
Figure 1. (A) PCR genotyping of Cables1 and Apc. In the upper panel, lanes 1, 2, and 3 represent the Cables1 wild-type, heterozygous, and homozygous genotypes, respectively. WT, wild-type fragment (~300 bp); KO, knockout fragment (~200 bp). In the lower panel, lanes 1 and 2 represent the ApcMin/+ and Apc wild type genotypes, respectively. WT fragment (~600 bp); ApcMin fragment (~340 bp). (B) Intact nuclear Cables1 expression by immunohistochemistry in Cables1+/+ ApcMin/+ mouse intestinal epithelial cells. (C) Loss of nuclear Cables1 expression by immunohistochemistry in Cables1−/−ApcMin/+ mouse intestinal epithelial cells. A few inflammatory cells retain Cables1 expression.
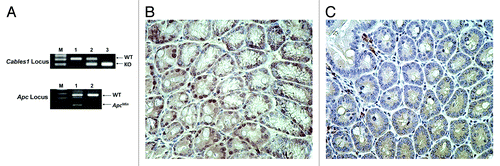
Figure 2. (A) Hematoxylin and eosin (H&E) stained small intestine from a Cables1+/+ApcMin/+ mouse with rare adenomas. (B) H&E stained small intestine from a Cables1−/− ApcMin/+ mouse with frequent adenomas. (C) An adenocarcinoma in the small intestine of a Cables1−/− ApcMin/+ mouse. Inset: higher magnification.
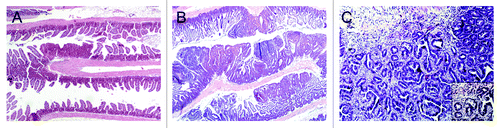
Table 1. Tumor burden in Cables1+/+ ApcMin/+ mice and Cables1−/− ApcMin/+mice
Figure 3. (A) Membranous and cytoplasmic β-catenin immunohistochemical staining was observed in the adenomas of Cables1+/+ ApcMin/+ mice, but there was minimal nuclear staining. (B) In the adenomas of Cables1−/− ApcMin/+ mice, there was nuclear immunopositivity for β-catenin. (C) A low proportion of epithelial cells were PCNA positive in adenomas from Cables1+/+ ApcMin/+ mice. (D) In the adenomas of Cables1−/− ApcMin/+ mice, a high proportion of epithelial cells were PCNA-positive by immunohistochemistry.
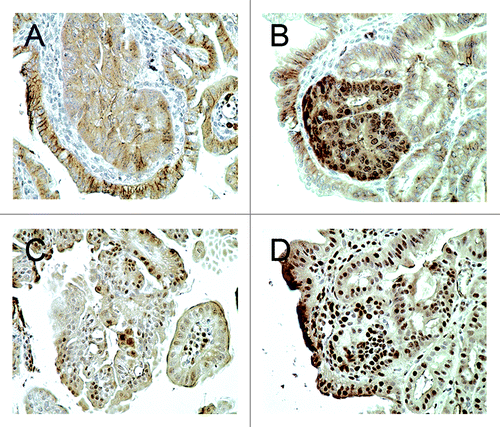
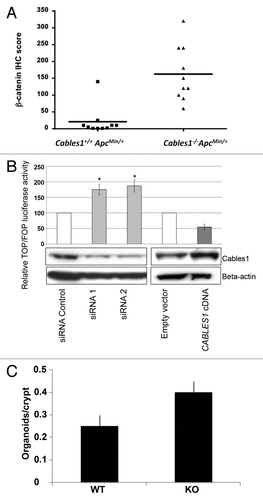
Figure 4. (A) β-catenin immunohistochemistry (IHC) scores of Cables1+/+ ApcMin/+ and Cables1−/− ApcMin/+ mice. A horizontal line indicates the median in each group. There was a significantly higher mean nuclear β-catenin IHC score (P = 0.002) in the Cables1−/− ApcMin/+ mice. (B) SW480 cells were transfected with either small interfering RNA (siRNA) targeting endogenous CABLES1 or with CABLES1 cDNA. Twelve hours later, cells were transfected with a β-catenin responsive (pGL3-OT) or a mutant (pGL3-OF) reporter construct and luciferase activity was measured. Results are expressed as the TOP/FOP ratio. The CABLES1 siRNA was associated with significantly increased TOP/FOP reporter activity compared with cells transfected with a control siRNA. Overexpression of Cables1 following CABLES1 cDNA transfection was associated with decreased TOP/FOP reporter activity compared with controls. Columns: average of at least two independent experiments; bars: standard error of mean (SEM). *P < 0.05 as compared with control cells. Protein levels of Cables1 were confirmed by western blot in lower panel. (C) Small intestinal crypts isolated from Cables1 knockout mice (KO) were capable of nearly 2-fold greater (P < 0.05) organoid formation than crypts from wild type mice (WT), indicating increased intestinal progenitor cell activity in the Cables1 knockout mice.