Figures & data
Figure 1. Impact of K-RAS activity on tumor cell clonogenicity. (A) The basal level of K-RAS-GTP was determined as described.Citation39 (B) Total cell lysates were subjected to sodium dodecyl sulfate-PAGE (SDS-PAGE). Following Ponceau staining, the expression level of K-RAS was analyzed by western blotting. Actin was detected as a loading control. (C) FaDu cells were transiently transfected with pEGFP-C1 empty vector or pEGFP/K-RAS(V12); 48 h after transfection, green fluorescent protein (GFP) expression was analyzed by fluorescent microscopy. (D) After microscopy analysis, the cells were lysed, and western blotting was performed. Following detection of K-RAS and P-Akt (S473), the blots were stripped and incubated with antibodies against GFP and Akt1. Actin was used as a loading control. The densitometric values represent the ratios of P-Akt (S473) to Akt1 normalized to 1 in the control vector-transfected cells. (E) FaDu cells were transiently transfected with empty vector or vector expressing K-RAS(V12); 48 h after transfection, the cells were plated for a clonogenic assay. Homozygous K-RAS(G12V) significantly increased PE. The data present the mean ± SD of 12 parallel experiments (*P < 0.05).
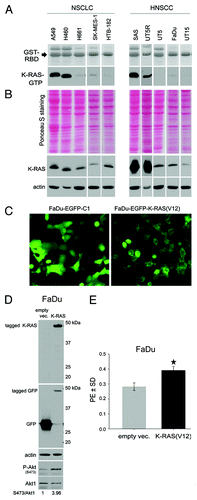
Figure 2. K-RAS activity is associated with erlotinib resistance and accompanied with increased autocrine production of AREG. (A and B) The effect of erlotinib on clonogenic activity was determined using a clonogenic assay. The data points shown represent the mean PE ± SD of at least 12 data from two independent experiments. The inhibition of clonogenic activity by erlotinib is dependent on the cell line (*P < 0.05; **P < 0.01; ***P < 0.001). (C) Cells were incubated in serum-free medium for 48 h, and the concentration of AREG was measured by ELISA. The data present the mean ± SD of 12 data from 4 independent cultures of SAS cells, 4 data from 2 independent cultures of UT5R, and 11 data from 4 independent cultures of UT5 cells (***P < 0.001).
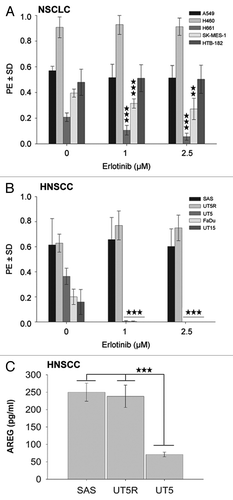
Figure 3. K-RAS knockdown sensitizes cells to erlotinib. (A) A549 and SAS cells were transfected with control (ctrl)-siRNA or K-RAS-siRNA. Two days after transfection, the efficiency of K-RAS-siRNA was analyzed by western blotting. (B) The cells were plated in 6-well plates for a clonogenic assay two days after transfection with the indicated siRNAs and then treated with erlotinib (1 µM) after 24 h. The histograms represent the mean PE ± SD of 12 parallel data in A549 cells and 18 data from two independent experiments in SAS cells (*P < 0.05).
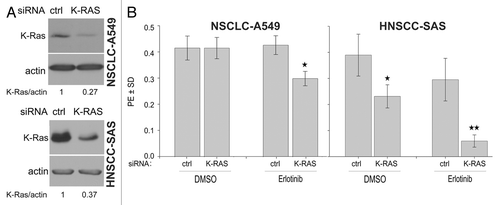
Figure 4. The clonogenic activity of tumor cells depends mainly on the activation of PI3K-Akt but not on the MAPK-ERK1/2 pathway. (A) Cells were treated or not with the MEK inhibitor PD98059 (20 µM) for 24 h, and the level of P-ERK1/2 and ERK1/2 was analyzed by western blotting. (B) Cells were plated in 6-well plates for a clonogenic assay and were treated with 20 µM of PD98059 after 24 h. (C) Cells were treated or not with the indicated concentrations of PI3K inhibitor PI-103 for 24 h. The phosphorylation levels of Akt were analyzed by western blotting using isolated protein samples; the blots were re-probed with an anti-Akt1 antibody. (D) Effect of PI-103 on PE was determined by a clonogenic assay. The data points represent the mean PE ± SD of at least 12 data from two independent experiments. The statistical analysis indicated a differential effect of PD98059 (B) and PI-103 (D) on the clonogenic activity of the tested cell lines (*P < 0.05; **P < 0.01; ***P < 0.001). The densitometric values in (A and C) represent the ratios of P-Akt/Akt1 and P-ERK1/2 to ERK1/2 normalized to 1 in the DMSO-treated controls.
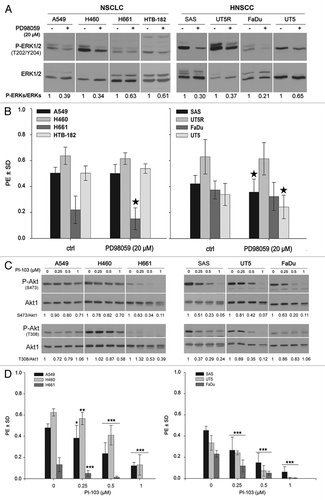
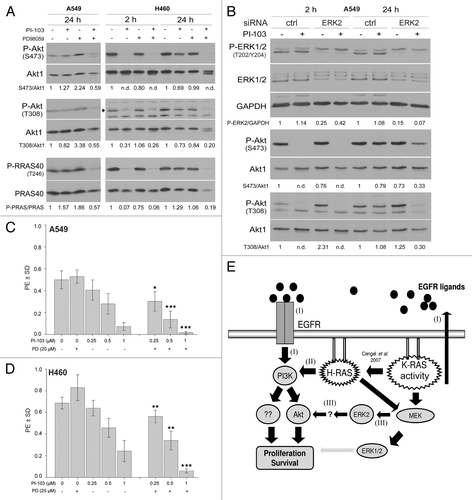
Figure 5. Long-term inhibition of EGFR and PI3K results in the reactivation of Akt. (A) A549 cells were lysed at 2 h and 24 h after treatment with or without the indicated concentrations of erlotinib. (B–D) Cells were treated with the indicated concentrations of PI-103; at 2 and 24 h after treatment, protein samples were isolated and subjected to SDS-PAGE. The levels of P-Akt (S473 and T308), P-GSK3α/β (S21/S9), and P-PRAS40 (T246) were analyzed by western blotting. The blots were stripped and incubated with antibodies against Akt1, GSK3α/β, and PRAS40. The densitometric values represent the ratios of P-Akt (S473 and T308)/Akt1 (A and B), P-PARA40/PRAS40 (B–D), and P-GSK3α/GSK3α (D) normalized to 1 in the corresponding controls. n.d., non-detectable.
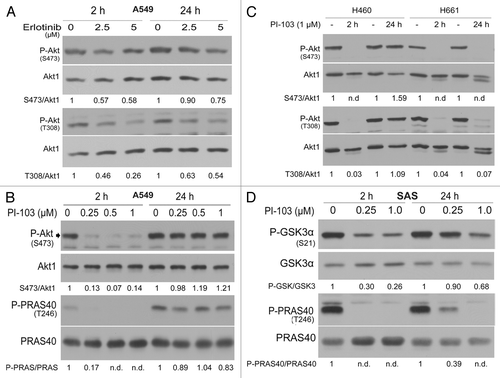
Figure 6. The ERK2-dependent reactivation of Akt in K-RASmut cells following long-term treatment with PI-103 improves clonogenic survival. (A) A549 and H460 cells were treated with PI-103 (1 µM) for the indicated times, and protein samples were isolated and subjected to SDS-PAGE. The levels of P-Akt (S473 and T308) and P-PRAS40 (T246) were detected by western blotting; the blots were stripped, and total proteins were detected. (B) Cells transfected with control-siRNA (ctrl) or ERK2-siRNA were treated with DMSO or PI-103 at 3 d after transfection; 24 h after treatment, protein samples were isolated and subjected to SDS-PAGE. The levels of ERK1/2, PDK1, and P-Akt (S473 and T308) were detected by western blotting; the blots were stripped and re-incubated with an anti-Akt1 antibody. GAPDH was used as a loading control. (C and D) Cells were plated in 6-well plates for a clonogenic assay; after 24 h, the cells were treated the indicated concentrations of MEK inhibitor PD98059 (PD), PI3K inhibitor PI-103 (PI), or combination of PI and PD. Colonies that formed after 10 d were counted, and PE was calculated and graphed. The data points shown represent the mean PE ± SD of 12 data from two independent experiments. The statistical analysis indicated that the combination of PI and PD significantly increased the anti-clonogenic activity compared with PI alone (*P < 0.05; **P < 0.01; ***P < 0.001). (E) A model illustrating the signaling pathways involved in proliferation and survival of tumor cells with K-RAS mutation or cells overexpressing K-RASwt. The densitometric values represent the ratios of P-Akt (S473 and T308)/Akt1, P-PARA40/PRAS40, and P-ERK2/GAPDH normalized to 1 in the corresponding controls. n.d., non-detectable.