Figures & data
Figure 1. PRISMA chart showing the trial exclusion and inclusion process in the metaanalysis. SEER, Surveillance, Epidemiology, and End Results.
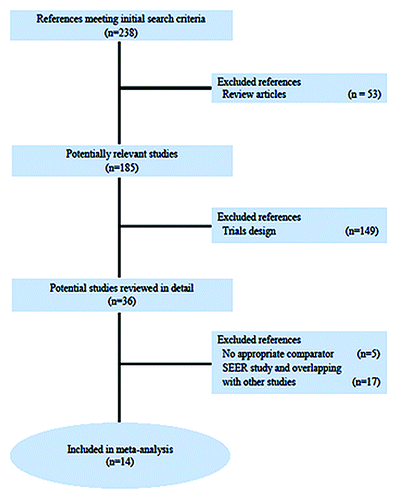
Table 1. Survival data extracted from analyzed clinical trials
Table 2. Response rate and toxicities data extracted from analyzed clinical trials
Table 3. Quality assessment
Figure 2. Comparison of OS and PFS, according to treatment line (A andB respectively) or platinum sensitivity (C and D respectively), between patients treated with a PLD-containing regimen vs. any other PLD-free schedule. Abbreviation: OS, overall survival; PFS, progression free survival; HR, hazard ratio; CPLD, carboplatin and pegylated liposomal doxorubicin; CP, carboplatin and paclitaxel; PLD pegylated liposomal doxorubicin; O, olaparib; C, carboplatin; G, gemcitabine; T, topotecan.
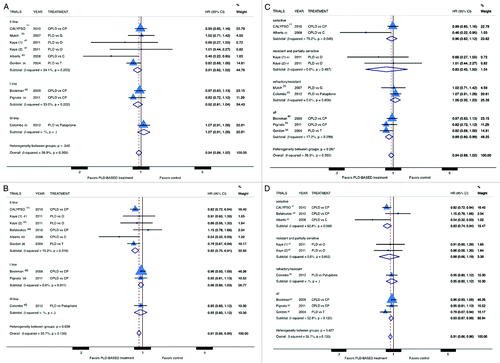
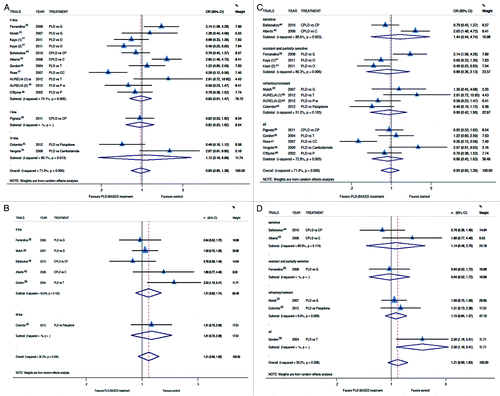
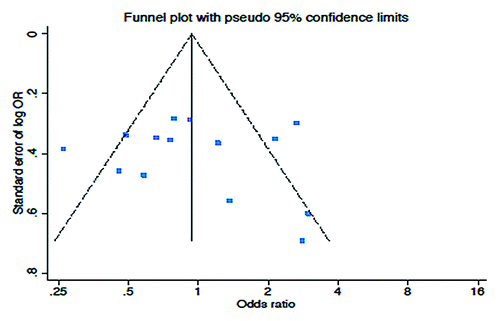
Figure 3. Comparison of RR and Ca125 response, according to treatment line (A and B respectively) or platinum sensitivity (C andD respectively), between patients treated with a PLD-containing regimen vs. any other PLD-free schedule. Abbreviation: OR, odds ratio; rr, risk ratio; CPLD, carboplatin and pegylated liposomal doxorubicin; CP, carboplatin and paclitaxel; PLD, pegylated liposomal doxorubicin; O, olaparib; C, carboplatin; G, gemcitabine; T, topotecan.