Figures & data
Figure 1. Established and primary GBM lines in response to PF299804 (dacomitinib). (A) Dacomitinib suppresses EGFR phosphorylation. All GBM cells were treated with dacomitinib for overnight (except that GBM8 cells were treated for 4 h), and cell lysates were analyzed by western blot. (B) Cell viability of GBM lines in response to 72 h treatment of different doses of dacomitinib. Mean ± SD. (C) Western blot analysis shows PLCγ1 activation is dependent on EGFR activation, and dacomitinib inhibits EGFR and its downstream effector PLCγ1 activation.
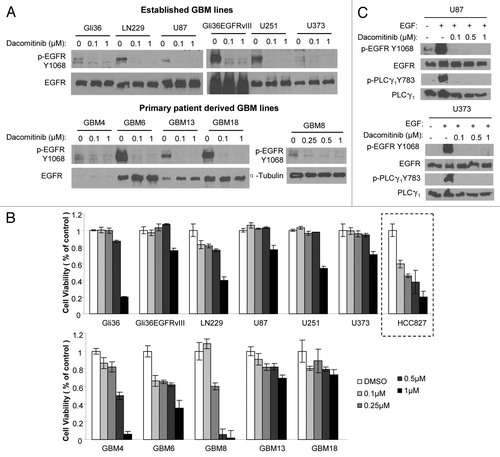
Table 1. Comparison of oncogenic RTKs pathways in GBM cell lines
Figure 2. Differential PI3K/mTOR signalings in response to dacomitinib in GBMs. Western blot analysis shows differential EGFR/PI3K/AKT/mTOR signalings in established GBM lines (A), patient-derived primary GBM lines (B), and engineered GBM4-EGFR or GBM4-EGFRvIII cells (C) in response to dacomitinib. All GBM cells were treated with indicated doses of dacomitinib and cell lysates were analyzed by western blot.
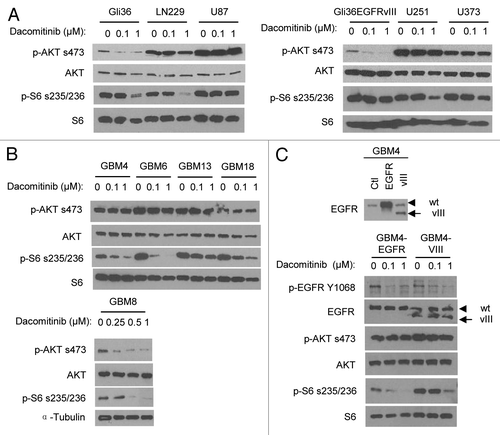
Figure 3. PI3K/mTOR dual inhibitor PF-05212384 inhibits EGFR-independent PI3K and mTOR activation in GBM cells. (A) Upper panel: GBM cells were exposed to indicate concentrations of PF-05212384 and analyzed by western blotting of PI3K and mTOR pathway markers. Bottom panel: Viability of cells in response to different doses of PF-05212384 for 48 h. (B) Western blot analysis of EGFR signaling in the presence of dacomitinib or/and PF-05212384 in GBM6 cells. (C) Western blot analysis of cleaved-PARP and cell viability of GBM6 cells in response to treatment of dacomitinib or/and PF-05212384 (96 h). Mean ± SD.
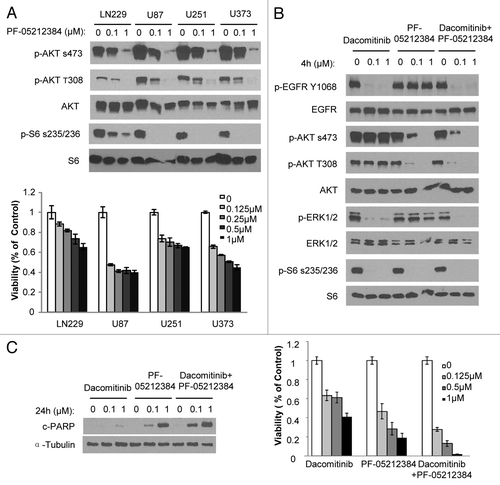
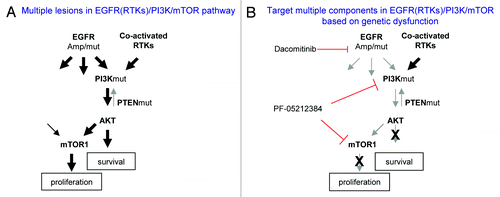
Figure 4. A simplified model of dysregulated EGFR (RTKs)/PI3K/mTOR pathway in GBM and corresponding target therapies. (A) Multiple lesions cause constitutive activation of EGFR (RTKs)/PI3K/mTOR pathway. (B) Targeting different components in EGFR(RTKs)/PI3K/mTOR pathway based on patient tumor genetic profile.