Figures & data
Figure 1 Optical phase contrast microphotographs showing the morphology tDPSCs and nDPSCs under phase contrast microscopy (x400) during the growth phase, day 3 (A and C) and at confluence, day 7 (B and D). Also shown is some immunopositive staining of tDPSCs and nDPSCs.
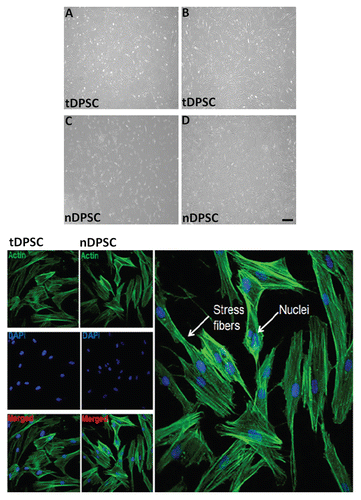
Figure 2 Relative fluorescent readings used to assess telomerase activity in tDPSCs and nDPSCs after the introduction of exogenous HTERT. The results are expressed as the means ± SD (n = 4, *p < 0.001).
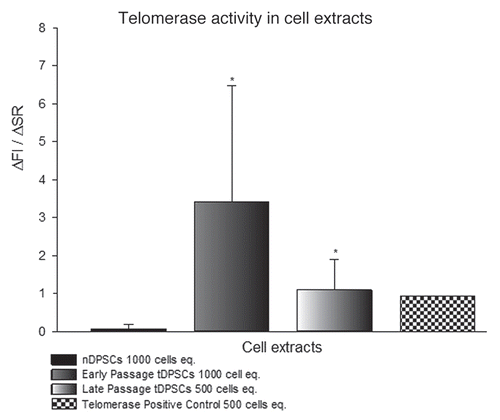
Figure 3 β-galactosidase staining in cultured human dental pulp stem cells. Cells were stained and photographed at 100x (A and C) or 200x (B and D) magnification. (A and B) Early passage nDPS C showing > 80% positivity and (C and D) Late passage tDPSCs showing < 10% positivity for β-galactosidase staining both depicted in the graph above. Positive staining disappeared after re-plating tDPSCs.
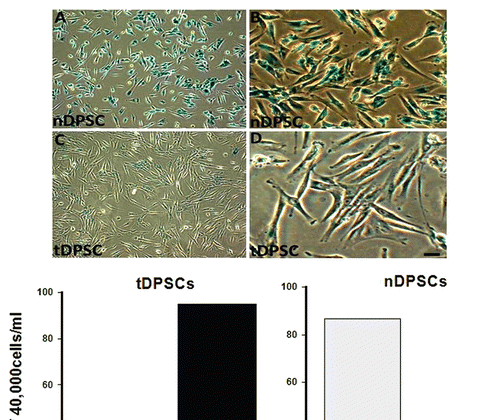
Figure 4 RT-PCR results for odontoblastic differentiation genes (ALP, cbfa-1, Col-1, DMP-1, DSPP, Osteopontin and Osteocalcin) and growth regulating factors p16, p21 and p53. Lane A, tDPSCs and Lane B, nDPSCs. GAPDH was run as an internal control and water as the negative control for both groups.
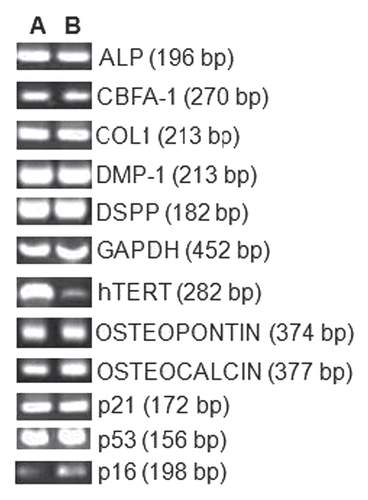
Figure 5 Analyses of cell proliferation using a DNA assay (A) and cell mineralization by way of an ALP assay (B) in tDPS C and nDPS C cell groups. The experiments were performed in triplicate. The values are means of the absorbance readings at 490 nm ± SD (n = 4, *p < 0.001).
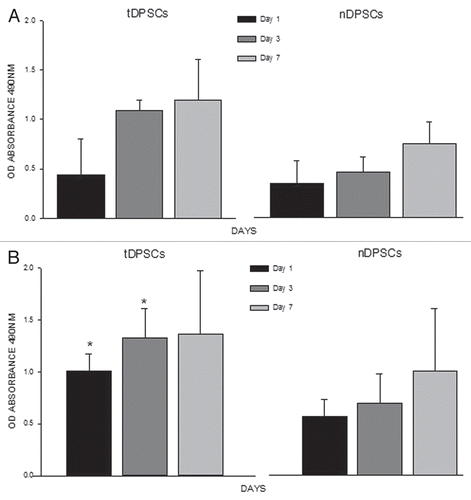
Table 1 Primer sequences and cycling conditions for the genes analyzed