Figures & data
Figure 1 Expression of CDK2, CDK1, and CDK5 in the melanoma progression pathway. Five-µm sections, prepared from cryopreserved tissue specimens representing normal skin, benign nevus, atypical nevus, melanoma in situ and VGP and MGP melanoma were probed with antibody to (A) CDK2, (B) CDK1 or (C) CDK5 and counterstained with hematoxylin.
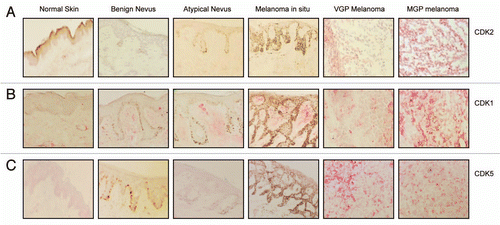
Figure 2 Expression of CDK2, pCDK2, CDK9, CDK5 and CDK1 in VGP and MGP melanoma cell lines, and in human neonatal melanocytes and skin fibroblasts. (A) Immunoblot analysis of CDK2, pCDK2, and CDK9 expression in the VGP melanoma cell line WM983-A (a), in MGP melanoma cell lines WM983-B (b), WM1158 (c), WM852 (d), WM266-4 (e), SK-MEL-28 (f), WM983-B (g), and in human foreskin-derived melanocytes (h), and human foreskin-derived fibroblasts (i). (B) Expression of CDK5 and CDK1 in MGP melanoma cell lines WM1158 (a), WM852 (b), WM266-4 (c), SK-MEL-28 (d), and WM983-B (e). For loading control, the immunoblots were probed with an antibody to α-tubulin.
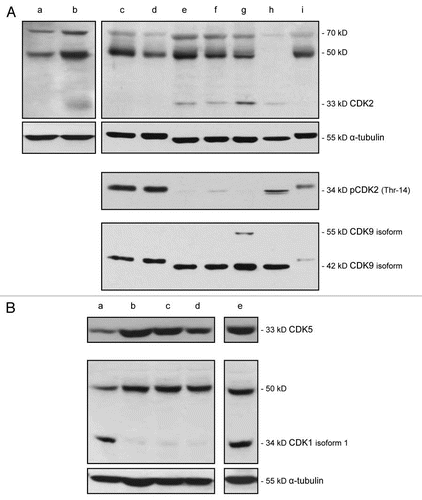
Figure 3 Impact of CDK inhibitor treatment of melanoma cells upon the expression of pCDK2, CDK9 and pRb. (A) pCDK2 (Thr-14), CDK9 and pRb (THR-821) immunoblot analysis of WM1158 MGP melanoma cells that received only DMSO (−) or were treated with 1 µM of the CDK inhibitor (+), SCH 727965 (Dinaciclib), for 24, 48, or 72 hr. Probing of the immunoblots with an antibody to α-tubulin served as loading control. (B) pCDK2 immunofluorescence analysis of MGP melanoma cells (WM1158) incubated in the presence of DMSO or treated with 1 µM of the CDK inhibitor (CDKI) for 24 hr. WM1158 cells that showed expression of pCDK2 are pseudocolored red. Nuclei, counterstained with fluorescent DAPI, are pseudocolored blue.
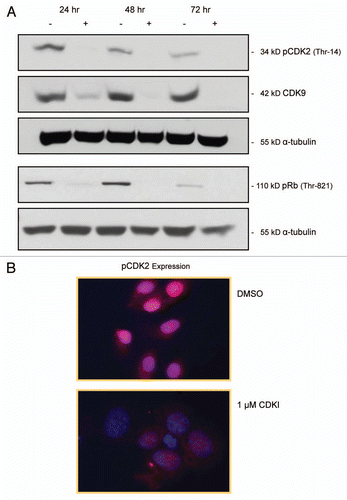
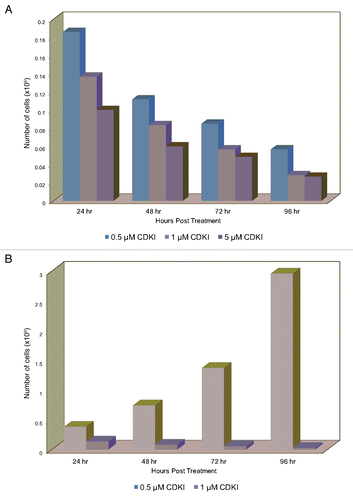
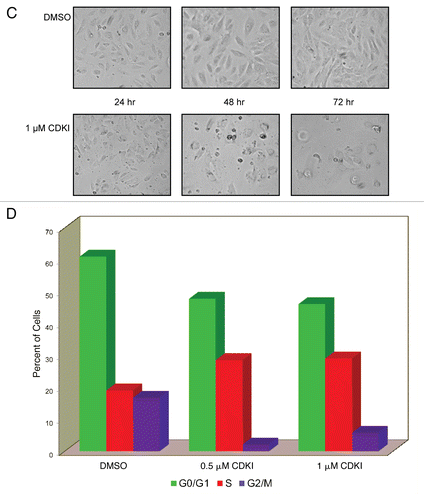
Figure 5 Inhibiting the function of CDKs in melanoma cells leads to melanoma cell apoptosis. (A) WM1158 MGP melanoma cells, treated with 1 µM of the CDK inhibitor (CDKI) for 48 or 72 hr, were analyzed by immunofluorescence-based TUNEL and likewise, Annexin V staining. WM1158 MGP melanoma cells that received only DMSO for 72 hr served as the control. Melanoma cells that had undergone apoptosis are pseudocolored red (TUNEL staining) or pseudocolored yellow (Annexin V staining). Fluorescent DAPI-counterstained nuclei are pseudocolored blue. (B) Immunoblot analysis of WM1158 MGP melanoma cells, treated with 1 µM, 2.5 µM, or 5 µM of the CDK inhibitor (CDKI) for 24, 48, or 72 hr, were probed with an antibody to c-PARP. WM1158 cells that received DMSO for 24, 48, and 72 hr served as the control. (C) After 24, 48, as well as 72 hr following treatment with 1 µM of the CDK inhibitor (CDKI, blue-colored bars), WM1158 MGP melanoma cells were labeled with Annexin V/Propidium Iodide and analyzed by flow cytometry. WM1158 cells that had received only DMSO (DMSO, grey-colored bars) served as the control. At each time point, the light-colored bars represent the percent of melanoma cells in the early stage of apoptosis, medium-colored bars the percent of melanoma cells in late-stage apoptosis and dark-colored bars the percent of necrotic melanoma cells.
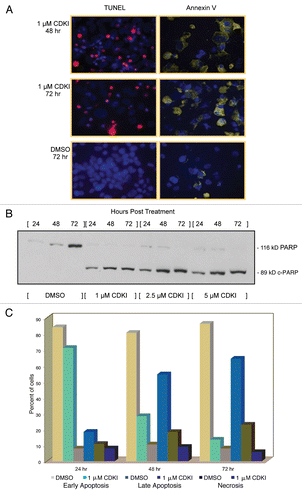
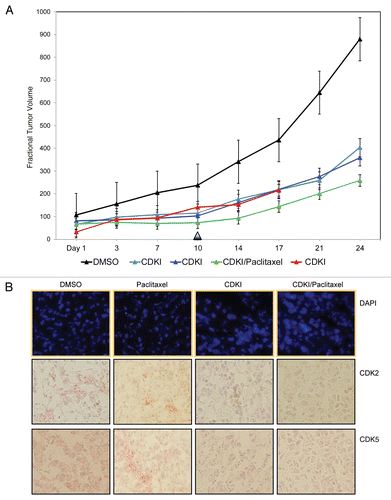
Figure 6 CDK inhibitor treatment of human melanoma xenograft-bearing nude mice. (A) Representative, individual examples of the growth rate of subcutaneous WM983-B MGP melanoma xenografts in nude mice that received the first i.p. injection of the CDK inhibitor (40 mg/kg) (CDKI; light blue-colored line; dark blue-colored line); CDK inhibitor (CDKI) (40 mg/kg) followed 24 hr later by i.p. injection of Paclitaxel (10 mg/kg) (CDKI/Paclitaxel: green-colored line); or only the CDK inhibitor delivery vehicle DMSO (DMSO: black-colored line), on the day (Day 1) a tumor had reached 5 mm in any direction. Subsequent i.p. injections were given on Day 3, 7 and 10. Following the last i.p. injections on Day 10 (indicated by the triangle), tumor volumes in these animals were further recorded on Day 14, 17, 21 and 24. The red-colored line depicts the growth rate of a WM983-B MGP melanoma xenograft in an animal that had been treated i.p. with 40 mg/kg of the CDK inhibitor on Day 1, 3, 7, 10, 14 and 17. (B) CDK2 and CDK5 immunohistochemical analysis of tissue sections prepared from WM983-B MGP melanoma xenografts that until Day 10 had been injected with only DMSO or only Paclitaxel, the CDK inhibitor (CDKI), or the CDK inhibitor combined with Paclitaxel (CDKI/Paclitaxel). Images of the fluorescent DAPI-stained or hematoxylin-counterstained and CDK2 or CDK5-stained tumor sections are depicted at 40X and 20X magnification, respectively.