Figures & data
Figure 1. ASAP interacts with p53. (A) Analysis of the interaction between ASAP and p53 by yeast two-hybrid screen. The yeast strain Y187 transformed with the pGBT11-ASAP hybrid expression vector was mated with the yeast strain CG1945 transformed with the pGAD-p53 hybrid expression vector. Positive clones exhibited His+ and LacZ+ phenotypes. (B) In vitro-translated p53 was incubated with equal amounts of purified GST, GST-ASAP or GST-ASAP-Cter immobilized on Glutathione-Sepharose beads in the presence of [35S] methionine. Bound [35S]-labeled p53 was visualized by SDS-PAGE and autoradiography (upper panel). Coomassie blue staining was used to assess protein loading (lower panel). GST-ASAP-Nter did not produce any protein using different conditions. (C) Schematic drawing of full-length p53 and deletion mutants used in this study. TA, transactivation domain; DB, DNA-binding domain; OD, oligomerization domain. Numbers indicate amino acid positions. (D) SaOS-2 cells were transiently transfected with the indicated combinations of expression plasmids. Twenty-four h after transfection, whole cell lysates were prepared and immunoprecipitated with the monoclonal anti-Flag antibody followed by immunoblotting with the monoclonal anti-p53 antibody (DO-1) (left, upper panel) and with the polyclonal anti-p53 antibody (FL-393) (right, upper panel). The left and right middle and lower panels show immunoblots of whole cell lysates performed with monoclonal (left) or polyclonal (right) anti-p53 or anti-ASAP antibodies.
![Figure 1. ASAP interacts with p53. (A) Analysis of the interaction between ASAP and p53 by yeast two-hybrid screen. The yeast strain Y187 transformed with the pGBT11-ASAP hybrid expression vector was mated with the yeast strain CG1945 transformed with the pGAD-p53 hybrid expression vector. Positive clones exhibited His+ and LacZ+ phenotypes. (B) In vitro-translated p53 was incubated with equal amounts of purified GST, GST-ASAP or GST-ASAP-Cter immobilized on Glutathione-Sepharose beads in the presence of [35S] methionine. Bound [35S]-labeled p53 was visualized by SDS-PAGE and autoradiography (upper panel). Coomassie blue staining was used to assess protein loading (lower panel). GST-ASAP-Nter did not produce any protein using different conditions. (C) Schematic drawing of full-length p53 and deletion mutants used in this study. TA, transactivation domain; DB, DNA-binding domain; OD, oligomerization domain. Numbers indicate amino acid positions. (D) SaOS-2 cells were transiently transfected with the indicated combinations of expression plasmids. Twenty-four h after transfection, whole cell lysates were prepared and immunoprecipitated with the monoclonal anti-Flag antibody followed by immunoblotting with the monoclonal anti-p53 antibody (DO-1) (left, upper panel) and with the polyclonal anti-p53 antibody (FL-393) (right, upper panel). The left and right middle and lower panels show immunoblots of whole cell lysates performed with monoclonal (left) or polyclonal (right) anti-p53 or anti-ASAP antibodies.](/cms/asset/0607ef77-bf1a-4bc9-99cf-51e5c5f52244/kccy_a_10920858_f0001.gif)
Figure 2. ASAP is transiently stabilized upon DNA damage in a p53-dependent manner. (A) U2OS cells were treated with 0.1 µM Doxorubicin (Dox) for the indicated times. Cells were fixed and stained with Propidium Iodide (PI) for Flow Cytometry analysis (FACS). (B) U2OS cells were treated with 0.1 µM Dox for the indicated times. (Left) Cell lysates were analyzed by western blotting with the indicated antibodies. α-Tubulin was used as a loading control. (Right) Total RNA was extracted, reverse transcribed (RT) and ASAP expression was determined by real-time quantitative PCR (qPCR). Results were normalized to GAPDH. Experiments were performed in triplicate. (C) Cell lysates of normal human fibroblasts Hs27 treated with 0.1 µM Dox for the indicated times were analyzed by western blotting with the indicated antibodies. Actin was included as a loading control. (D) Cell lysates of U2OS cells treated with 0.1 µM or 1 µM Dox for 8 h were analyzed by western blotting using anti-ASAP and -p53 antibodies. α-Tubulin was included as a protein loading control. (E) Total cell extracts from U2OS cells treated, or not, with 0.5 µM Dox for 4 h were immunoprecipitated using pre-immune rabbit serum or rabbit ASAP anti-serum. Precipitates were analyzed by immunoblotting with antibodies against ASAP and p53. The input loaded was 10% of the IP.
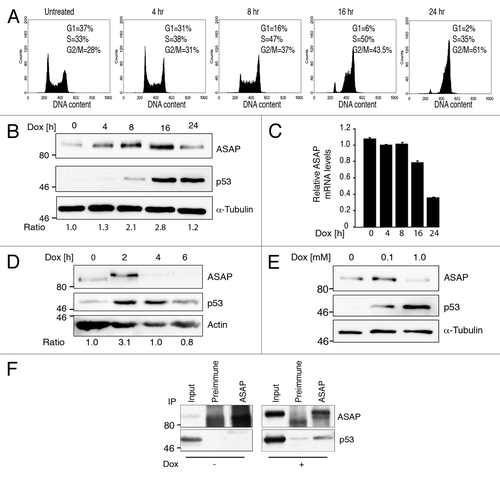
Figure 3. ASAP contributes to p53 stabilization. (A) p53−/− SaOS-2 cells were co-transfected with empty pcDNA3 vector, Flag-ASAP (0.1, 0.3, 0.5 µg) or p53 (0.02 µg) as indicated. Cells lysates were collected 24 h post-transfection and analyzed by western blotting with the indicated antibodies (anti-Flag for ASAP detection). Actin was used as a loading control. (B) SaOS-2 cells were transfected with expression plasmids encoding empty vector, Flag-ASAP or p53 as indicated. Twenty-four h post-transfection, cells were lysed for RNA isolation. p53 expression was analyzed by RT-qPCR. Results were normalized to GAPDH mRNA. Experiments were performed in triplicate. (C) SaOS-2 cells were transiently transfected with constructs encoding p53, Flag-HA-ASAP or Flag-ASAP-Nter (amino acids 412–647). Lysates were analyzed by immunoblotting with anti-ASAP, -p53 and -α-Tubulin antibodies. Asterisks (*) indicate non-specific bands. (D) SaOS-2 cells were transiently transfected with expression plasmids encoding p53, Flag-ASAP or Flag-ASAP-Cter (amino acids 1–421). Cell lysates were analyzed by immunoblotting using anti-ASAP and anti-p53 antibodies. α-Tubulin was included as loading control. (E) 24 h after transfection with Flag-ASAP or empty vector, U2OS cells were treated with cycloheximide (CHX) for the indicated times, harvested and analyzed by western blotting with anti-ASAP and -p53 antibodies. α-Tubulin was used as a loading control. Band intensities were quantified with ImageJ and p53 concentration normalized to α-Tubulin (p53/α-Tubulin) is represented in the right panel. (F) SaOS-2 cells were transfected with 0.5 µg of luciferase reporter plasmids under the control of the PG13 (left panel) or MDM2 promoter (right panel) alone, or with 0.02 µg p53 and increasing amounts (0.5, 1 and 1.5 µg) of Flag-ASAP expression vector (which corresponds to the transfection conditions used in 3A). Twenty-four h after transfection, firefly luciferase activity was determined in cell lysates and normalized against Renilla luciferase activity. (G) SaOS-2 cells were transiently transfected with a luciferase reporter construct driven by the PG13 promoter and a combination of plasmids as indicated. Twenty-four h post-transfection, firefly luciferase activity was determined in cell lysates and normalized against Renilla luciferase activity. Results are presented as mean SD of three independent experiments. Asterisks (*) indicate a significant increase in pG13 luciferase activity in cells transfected with p53 and Flag-HA-ASAP-Cter compared with cells transfected with p53 alone (p < 0.00002).
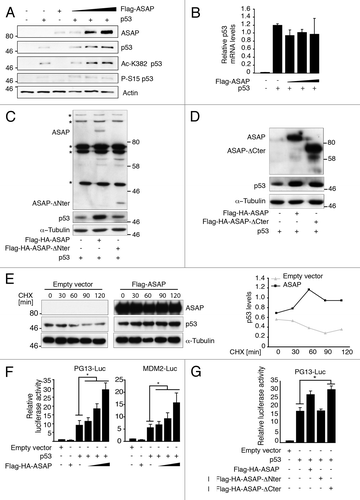
Figure 4. MDM2 p53 target is stabilized upon ASAP induction. (A) SaOS-2 cells were transiently transfected with the indicated constructs and analyzed by western blotting using anti-ASAP, -p53 and -MDM2 antibodies. α-Tubulin was used as a loading control. (B) U2OS cells were transiently transfected with GL2 or ASAP siRNA and, 48 h later, treated or not (-) with 0.1 µM Dox for 4 h. Total RNA was extracted and MDM2 and ASAP expression was analyzed by real-time quantitative PCR (RT-qPCR). Results were normalized to GAPDH mRNA. Experiments were performed in triplicate. (C) U2OS cells were transiently transfected with GL2 or ASAP siRNA and, 48 h later, treated or not (-) with 0.1 µM Dox for 4 h. After lysis, western blot analysis was performed with the indicated antibodies. Actin was used as a loading control.
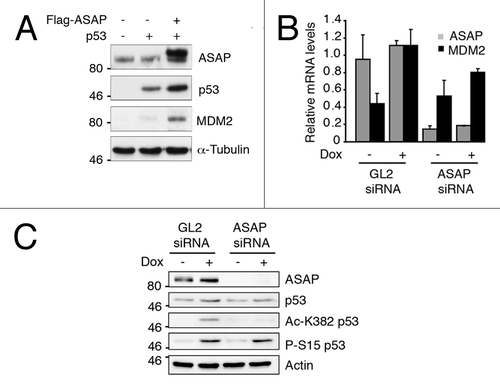
Figure 5. ASAP favors p300-dependent p53 acetylation. (A) SaOS-2 cells were transiently transfected with p53, Flag-ASAP and HA-p300 expression plasmids for 24 h and cell extracts were analyzed by western blotting with the indicated antibodies. α-Tubulin was included as loading control. (B) SaOS-2 cells were transiently transfected with 0.5 µg of luciferase reporter plasmids under the control of the MDM2 (left panel) or p21 promoter (right panel) alone, or with 0.02 µg p53, 1 µg Flag-ASAP or HA-p300 expression plasmids as indicated. Twenty-four h after transfection, firefly luciferase activity was determined in cell lysates and normalized against Renilla luciferase activity. (C) Total cell extracts from U2OS cells treated or not (-) with 0.1 µM Dox for 8 h were immunoprecipitated using mouse anti-IgG or monoclonal anti-ASAP antibodies. Precipitates were analyzed by immunoblotting with antibodies against ASAP and p300. The input loaded was 10% of the IP.
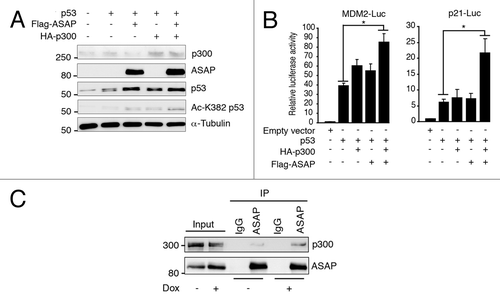
Figure 6. ASAP inhibits MDM2-mediated p53 ubiquitination and degradation. (A) p53−/− SaOS-2 cells were transiently transfected with empty pcDNA3 vector, HA-MDM2, p53 and increasing amounts of Flag-ASAP (1 and 1.5 µg) expression plasmids. Immunoblotting was performed 24 h later with the indicated antibodies. Asterisks (*) indicate non-specific bands. α-Tubulin was included as loading control. (B) HEK293T cells were transiently transfected with p53 (500 ng), Flag-MDM2 (200 ng), HA-Ubiquitin (1.5 µg) and Flag-ASAP (6 and 7 µg) plasmids for 48 h, treated with 40 µM MG132 for 3 h and harvested. Cell lysates were immunoprecipitated with anti-p53 antibodies. Ubiquitinated forms of precipitated p53 were visualized using an anti-HA antibody. Western blotting of cell lysates performed with the indicated antibodies to detect the transfected proteins is shown below. (C) Cell lysates processed in were used in parallel for this experiment: HEK293T cells were transiently transfected with p53 (500 ng), Flag-MDM2 (200 ng), HA-Ubiquitin (1.5 µg) and Flag-ASAP (6 and 7 µg) plasmids for 48 h, treated with 40 µM MG132 for 3 h and harvested. Cell lysates were immunoprecipitated with anti-MDM2 antibodies. Ubiquitinated forms of precipitated MDM2 were visualized using an anti-HA antibody. Western blotting of cell lysates performed with the indicated antibodies to detect the transfected proteins is shown. (D) U2OS cells were transiently transfected with 6xHis-Ubiquitin (5 µg) and Flag-ASAP (7 µg) plasmids for 48 h, treated with 40 µM MG132 for 3h. Ni+-purified 6xHis-Ub-conjugated proteins were probed either for the presence of endogenous ubiquitinated p53 using an anti-p53 (DO-1) (left) or an anti-His antibody (right). Cell lysates were analyzed by western blotting with the indicated antibodies (Input).
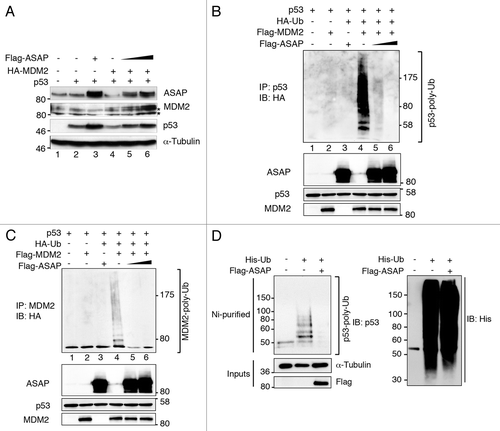
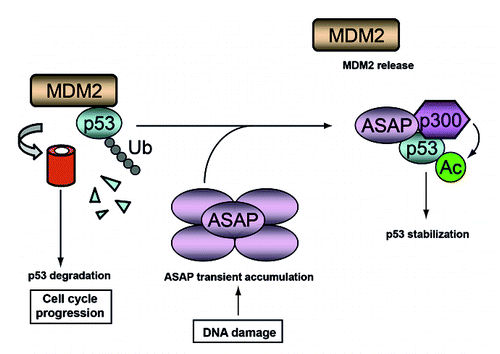
Figure 7. A schematic model describing the postulated ASAP-mediated stabilization of p53 following DNA damage. In growing cells, MDM2 degrades p53 to allow cell cycle progression. Upon DNA damage, ASAP is transiently accumulated and interacts with p53 to promote its stabilization by interfering with MDM2-p53 complex formation and suppressing its MDM2-mediated ubiquitination, thus facilitating its p300-mediated acetylation.