Figures & data
Figure 1. As3+ induces EZH2 S21 phosphorylation and activation of JNK and Akt. (A) As3+ induces EZH2 S21 phosphorylation and activation of JNK and Akt in a dose-dependent manner. BEAS-2B cells were treated with the indicated doses of As3+ for 4 h and analyzed by western blotting using the indicated antibodies. H3K27me3, trimethylation of lysine 27 on histone H3; pEZH2, S21-phosphorylated EZH2. (B) Time-course studies of As3+-induced EZH2 S21 phosphorylation, activation of JNK and Akt. BEAS-2B cells were treated with 20 μM As3+ for the indicated times. Total cellular proteins were prepared for western blotting at the end of each treatment. (C) As3+-induced EZH2 S21 phosphorylation requires Akt activation. BEAS-2B cells were transfected with a negative control, 50 nM control siRNA (siCon) or 50 nM Akt siRNA (siAkt) as described in the Materials and Methods. Forty-eight hours after transfection, the cells were treated with 20 μM As3+ for 2 h. At the end of the cell culture period, cell lysates were prepared for determination of EZH2 S21 phosphorylation (pEZH2) and Akt activation by western blotting. Data are representative of at least three independent experiments.
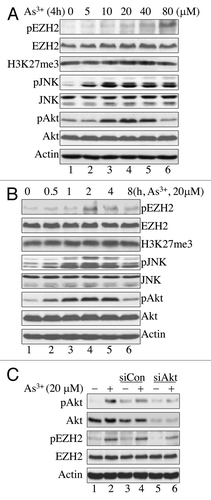
Figure 2. EZH2 phosphorylation at S21 is JNK-dependent. (A) JNK inhibition blocked As3+-induced Akt activation and EZH2 S21 phosphorylation. BEAS-2B cells were cultured in the presence or absence of 20 μM SP600125 (SP), a JNK inhibitor for 4 h and then with 20 μM As3+ for an additional 2 h. (B) siRNA-mediated silencing of JNK1 diminished As3+-induced EZH2 S21 phosphorylation and activation of STAT3 and Akt. BEAS-2B cells were transfected with a negative control, 50 nM control siRNA (siCon), 50 nM JNK1 siRNAb (siJNK1b) or 50 nM JNK1 siRNAc (siJNK1c). Cell lysates were prepared for western blotting after treatment with or without 20 μM As3+ for an additional 2 h. Data are representative of three or four independent experiments.
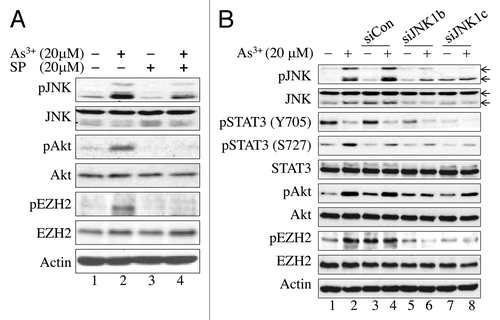
Figure 3. Involvement of STAT3 in As3+-induced Akt activation and EZH2 S21 phosphorylation. (A) BEAS-2B cells were pretreated with a negative control, 20 μM STAT3 Inhibitor V (STAT3 In) or DMSO as a vehicle control for 4 h. The cells were then incubated in the presence or absence of 20 μM As3+ for an additional 2 h. (B) As3+ induces STAT3 reporter gene activity. BEAS-2B cells were transfected with a STAT3 reporter system containing a 40:1 mixture of a STAT3 firefly luciferase reporter vector and a renilla luciferase reporter vector. Twenty-four hours after transfection, the cells were treated with the indicated concentrations of As3+ for 6 h. Dual-luciferase activity was determined at the end of the cell culture period. The right panel shows a time-course study of As3+-induced STAT3 reporter gene activity. *: p < 0.05; **: p < 0.005. (C) As3+ reduces immunofluorescent signal of Y705-phosphorylated (pY705) STAT3. (D) As3+ induces nuclear accumulation of the S727-phosphorylated STAT3. Data are representative of three experiments.
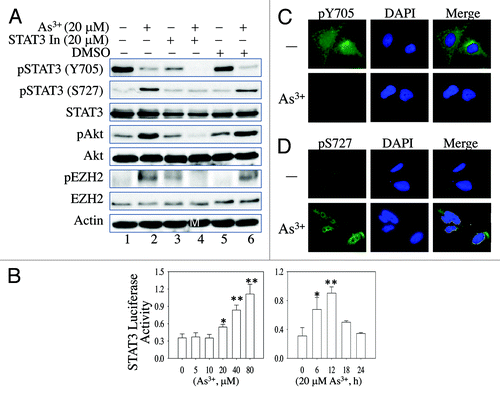
Figure 4. MiR-21 enhances As3+-induced Akt activation by downregulating Spry2. (A) As3+ induces miR-21 expression in BEAS-2B cells. The cells were cultured in the presence or absence of 20 μM As3+ for 6 h. The expression levels of miR-21 were determined by real-time PCR. (B) Overexpression of miR-21 precursor activates Akt and induces EZH2 S21 phosphorylation. Cells were transfected with 100 nM control miRNA or miR-21 precursor. EZH2 phosphorylation and Akt activation were determined by western blotting 24 h after transfection. (C) BEAS-2B cells were transfected with 30 nM anti-miR-21 (miR-21 inhibitor). Twenty-four hours later, the cells were incubated in the presence or absence of 20 μM As3+ for 2 h. EZH2 S21 phosphorylation and Akt activation were determined in cell lysates by western blotting. (D) Overexpression of miR-21 precursor downregulates Spry2. BEAS-2B cells were transfected with 100 nM control miR or 100 nM miR-21 precursor. The protein levels of PTEN, PCDC4 and Spry2 were determined 24 h after transfection. Data are representative of three experiments.
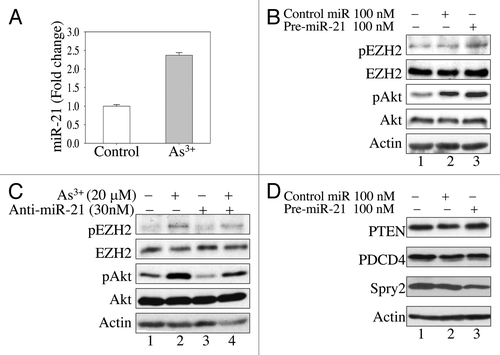
Figure 5. As3+ induces cytosolic localization of S21-phosphorylated EZH2. (A) Immunofluorescent staining of EZH2 in control cells or cells treated with 20 μM As3+ for 2 h. (B) Immunofluorescent staining of S21-phosphorylated EZH2 (pEZH2) in cells cultured in the presence or absence of 20 μM As3+ for 2 h. Note the punctuation pattern of pEZH2 in the cytoplasm. (C) Nuclear or cytosolic localization of EZH2 and S21-phosphorylated EZH2 as determined by cellular fractionation. The purity of cytosolic fraction and nuclear fraction was validated by detecting GAPDH and Histone H3, respectively. The arrows indicate pEZH2; the blank triangle on the light of the top panel indicates non-specific bands in the nuclear fractions. Data are representative of three experiments.
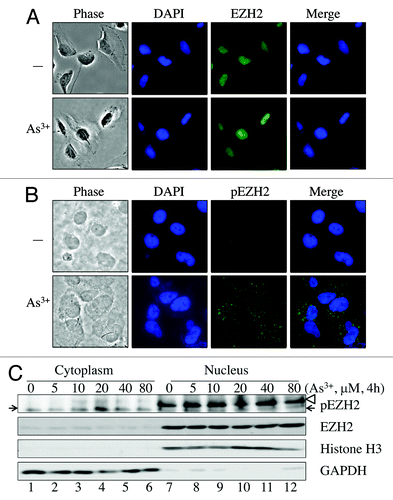
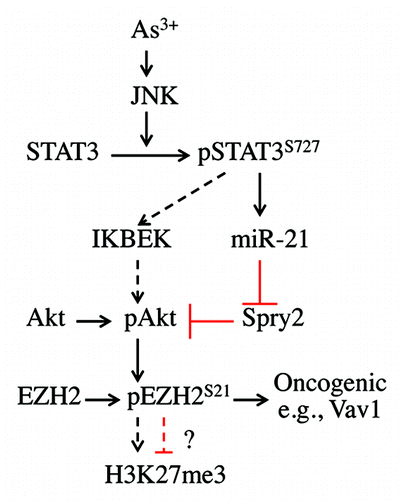
Figure 6. Signaling cascade of As3+-induced EZH2 S21 phosphorylation. In response to As3+ treatment, activated JNK phosphorylates STAT3 at S727 to enhance the transcriptional activity of STAT3, leading to increased expression of miR-21, which, in turn, downregulates the expression of Spry2, a negative regulator of Akt signaling. The activated Akt phosphorylates EZH2 at S21, possibly causing the dissociation of PRC2 complex from chromatin and the cytosolic localization of S21-phosphorylated EZH2. JNK-activated STAT3 may also upregulate the expression of IKBKE, which can directly phosphorylate and activate Akt (dashed arrows), leading to EZH2 S21 phosphorylation. Following Akt-dependent phosphorylation on S21, the phosphorylated EZH2 may weaken H3K27me3 in nuclei or, alternatively, activate oncogenes, such as Vav1, as previously suggested, in the cytoplasm.