Figures & data
Figure 1. Bioinformatic analysis of the potential Atg10 homolog in S. pombe. CLUSTAL sequence alignment of Atg10 family members from human (HsAtg10), Caenorhabditis elegans (CeAtg10) and S. cerevisiae (ScAtg10), together with the predicted protein sequence of Spac227.04 (SpAtg10) is shown. Asterisks indicate identical amino acids shared by all the proteins; a colon, a highly similar substitution; full stop, a similar substitution. Bold indicates an amino acid that is found in at least three of the Atg10 proteins and ΨHPC motifs are shown in red.

Table 1. Open reading frames (ORFs) in S. pombe that contain the ΨHPC motif
Figure 2. A GFP release assay can be used to assess autophagy. (A and B) Cells lacking auxotrophic markers and expressing either untagged Atg8 (wt; SW576) or Atg8 tagged with GFP at the N terminus (Atg8-GFP; JT268) were incubated in minimal medium containing a nitrogen source (EMM) or minimal medium lacking a nitrogen source (EMM-N) in the presence or absence of 1 mM PMSF as indicated for 20 h at 30°C. Cell extracts were prepared and analyzed by western blotting using anti-GFP antibodies. The Atg8-GFP fusion protein and free GFP released during autophagy are indicated.
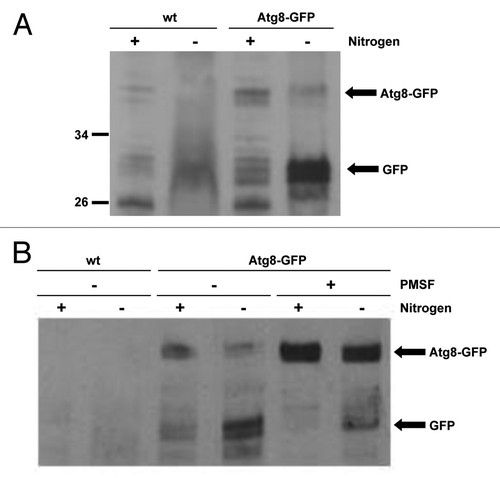
Figure 3. SpAtg10 is not essential for autophagy in S. pombe. (A) To investigate the potential role of SpAtg10 in autophagy, Atg8-GFP Δatg12 (MF43) and Atg8-GFP Δatg10 (MF31) cells lacking auxotrophic markers were incubated in EMM or EMM-N for 20 h at 30°C. Cell extracts were prepared and GFP release from the Atg8-GFP fusion protein was analyzed by western blotting using anti-GFP antibodies. The Atg8-GFP fusion protein and free GFP released during autophagy are indicated. (B) To determine whether SpAtg10 and/or Atg12 are required for the long-term survival of S. pombe cells during nitrogen starvation, wild-type (SW576), Δatg12 (MF13) and Δatg10 (MF15) cells were incubated in EMM-N medium at 30°C. Growth medium was removed and replaced with fresh medium every 3 d. Cells were removed from the EMM-N media and spotted onto YE5S plates after the indicated times. YE5S plates were incubated at 30°C for 3 d.
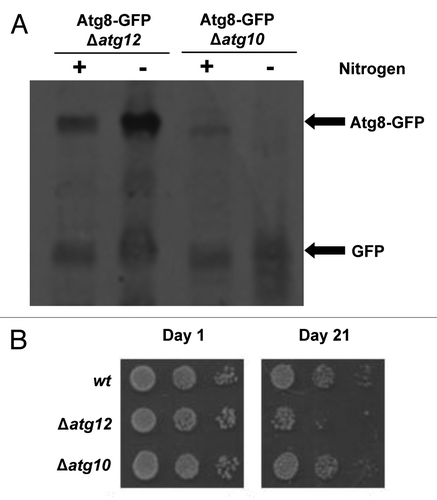
Figure 4. SpAtg10 but not Atg12 is essential for normal cell cycle progression. (A and B) Cells of mid-log-phase-growing cultures of wild-type (SW576), Δatg10 (MF15) and Δatg12 (MF13) strains, growing in liquid YE5S media at 30°C, were analyzed by (A) DIC microscopy and (B) a coulter counter. Growth curves were performed three times and error bars indicate the SEM. (C) Mid-log-phase-growing wild-type (CHP429) and Δatg10 (MF78) cells were fixed and spindle organization visualized by indirect immunofluorescence and nuclei visualized by DAPI staining. Representative images show cells of each strain at different stages of mitosis. In (A and C), the abnormal morphologies (small protrusions) of Δatg10 cells are indicated (arrows).
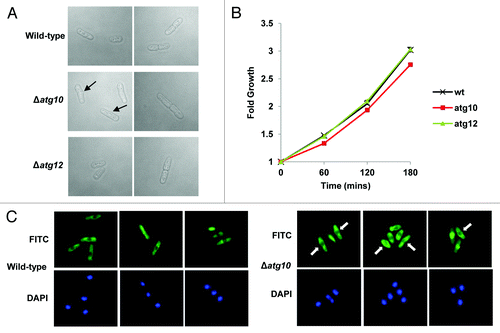
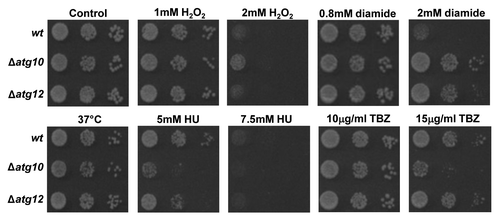
Figure 5. Loss of SpAtg10 affects the sensitivity of cells to drugs that perturb cell cycle progression. To investigate the potential role of SpAtg10 and the autophagy pathway in cell cycle processes, the sensitivity of wild-type (wt; SW576), Δatg10 (MF15) and Δatg12 (MF13) cells to different conditions known to affect cell cycle processes and checkpoints were examined. Ten-fold serial dilutions of mid-log-phase-growing cultures were spotted onto YE5S plates containing the indicated concentrations of H2O2, diamide, HU or TBZ. Plates were incubated at 30°C for 3 d. To investigate growth at 37°C cells were spotted onto a YE5S plate that was incubated at 37°C for 3 d.