Figures & data
Figure 1. Overexpressed Yen1-GFP suppresses MMS hypersensitivity of the mms4Δ yen1Δ strain. Overnight cultures of the strains FM1292 (cdc15-2) and FM1272 (cdc14-1) plus their derivatives carrying mms4Δ, mms4Δ yen1Δ, GAL:YEN1:GFP SPC42:RedStar, and mms4Δ GAL:YEN1:GFP SPC42:RedStar were serial diluted and plated onto 4 different plates; YP glucose, YP galactose, and either media further supplemented with MMS 0.004% (v/v). Pictures to determine ability to grow were taken after 5 d of incubation at 25 °C.
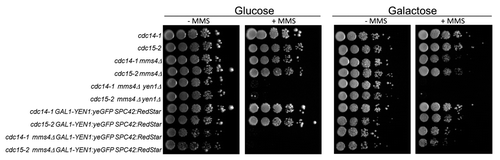
Figure 2. Yen1 re-localizes back to the nucleus in early anaphase in a Cdc14-dependent manner. Overnight YP raffinose cultures of the strains FM1799 (cdc15-2 GAL:YEN1:GFP SPC42:RedStar) and FM1804 (cdc14-1 GAL:YEN1:GFP SPC42:RedStar) were first arrested in G1 with αF for 3 h in the growth media. Next, galactose 2% (w/v) was added and the culture was left blocked in G1 for another 2 h. They then were split into 2 fresh YP glucose media, one of them containing MMS 0.004% (v/v), and finally released into a synchronous cell cycle at 37 °C for 4 h. (A) Representative micrographs of z-stack maximum projections from the G1 blocks after 2 h of galactose addition (G1 arrest), and about 100–120 min after the G1 release at 37 °C with or without MMS. The label (m) points to an S/G2 cell with an unaligned ~2 μm spindle (i.e., distance between SPBs) and no nuclear Yen1-GFP signal; the label (a) points to examples of cells in early anaphase according to spindle orientation and length, all with a visible nuclear Yen1; and the label (t) points to a cell already blocked in telophase with a much fainter nuclear Yen1 signal. (B) Percentage of cells with nuclear Yen1-GFP relative to the distance between SPBs (mean ± SEM, n = 3). Only budded cells with 2 SPBs were counted. (C) A sample from the FM1799 G1-to-telophase time course in MMS was treated with DAPI to address if foci colocalized with nuclear DNA. The label (f) points to an example of a cell in anaphase with foci within the main nuclear DNA mass, whereas the label (f') points to a cell in anaphase with foci located between the 2 segregated nuclear masses. BF, bright field.
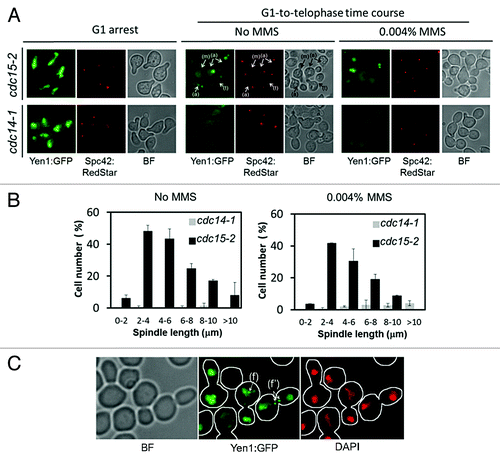
Figure 3. Yen1 nuclear relocalization in anaphase is dynamic and governed by the CDK/Cdc14 balance. Overnight YP raffinose cultures of the strains FM1799 (cdc15-2 GAL:YEN1:GFP SPC42:RedStar) and FM1804 (cdc14-1 GAL:YEN1:GFP SPC42:RedStar) were first arrested in G2 with nocodazole (Nz) for 3 h. Next, galactose 2% (w/v) was added, and the culture was left blocked in G2 for another 90 min. Next, Nz was removed from the media at the same time that glucose was added and the culture shifted to 37 °C. Cells were incubated in this condition for 90 min (G2-to-telophase cell cycle) before shifting the temperature to 25 °C and adding αF (Telophase-to-G1 cell cycle). (A) Representative micrographs of z-stack maximum projections from the different cell cycle blocks and/or time points of the corresponding releases. (B) Percentage of cells with nuclear Yen1-GFP at the different cell cycle blocks and/or time points of the corresponding releases. (C) Western blot against Yen1-GFP during the G2-to-telophase and telophase-to-G1 cell cycles. (D) Overnight YP raffinose cultures of the strains FM1799 (cdc15-2 GAL:YEN1:GFP SPC42:RedStar) and FM1850 (cdc15-2 GAL-CDC14 GAL:YEN1:GFP SPC42:RedStar) were first arrested in G2 with nocodazole (Nz) for 3 h. Next, galactose 2% (w/v) was added while the cultures were left blocked in G2 for another 90 min. BF, bright field.
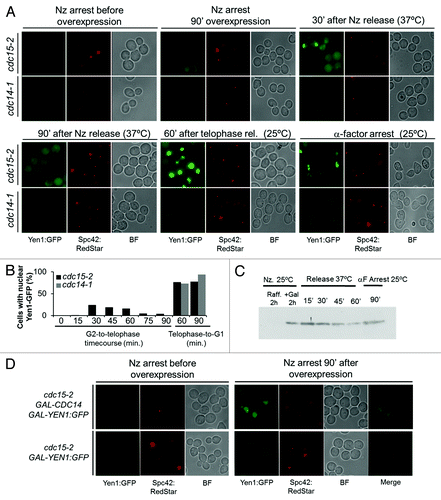
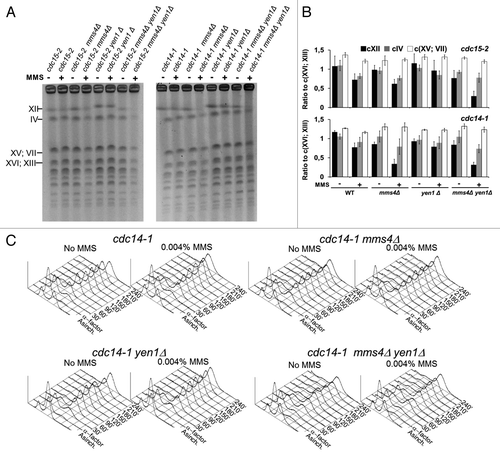
Figure 4. Yen1 and Cdc14 are epistatic in resolving Joint Molecules in the absence of Mus81-Mms4. Overnight YP glucose cultures of the strains FM1292 (cdc15-2) and FM1272 (cdc14-1), knocked out single mutant derivatives of them for MMS4 and YEN1, and the double mutants mms4Δ yen1Δ were first arrested in G1 at 25 °C. They were then split into 2 media, one of them containing MMS 0.004% v/v, and finally released into a synchronous cell cycle at 37 °C for 4 h. (A) PFGE of all yeast chromosomes at the telophase blocks. Minus sign, no MMS; plus sign, MMS 0.004% v/v. (B) Quantitation of the electrophoretic bands for the large chromosomes relative to the middle-sized chromosome pair XVI/XIII (mean ± SEM, n = 3). (C) Flow cytometry analysis of the DNA content of the G1-to-telophase time-course for the cdc14-1 strains.