Figures & data
Figure 1. KIBRA homologs and binding partners. The sequence similarity of each domain to human KIBRA is shown as a percentage. The arrowhead indicates the site of the serine residue that can be phosphorylated by aurora kinase.
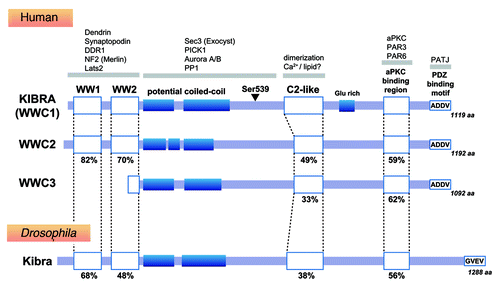
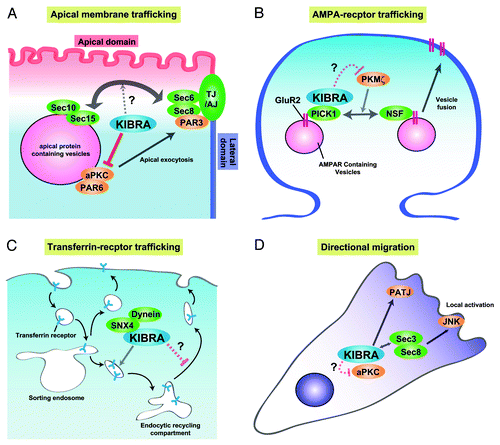
Figure 2. Models of the molecular mechanism of KIBRA in regulating membrane trafficking. (A) In epithelial cells, KIBRA suppresses the kinase activity of aPKC, resulting in suppression of the trafficking of apical-containing vesicles to the cell surface or cell-cell contact site. Association between KIBRA and the exocyst complex can influence this process. (B) In neurons, KIBRA associates with PICK1 and suppresses recycling of the AMPA receptor subunit, GluR2, to the cell surface. PKMζ enhances GluR2 surface expression via regulating the NSF-mediated release of GluR2 from PICK1. KIBRA may inhibit the kinase activity of PKMζ, leading to suppression of GluR2 recycling. (C) In endosomal trafficking of TfnR, KIBRA does not affect internalization, but affects trafficking from the endosome sorting compartment to the endosomal recycling compartment. (D) In directional migration, KIBRA can affect vesicular trafficking through interaction with the exocyst complex and aPKC.