Figures & data
Figure 1.Schematic illustration of the Dab2 and AP-2 interactions during clathrin-mediated endocytosis. Dab2 and AP-2 mediate assembly of the clathrin coat and recruitment of transmembrane cargo proteins for incorporation into clathrin coated pits (CCPs). The Dab2 DH domain interacts with the NPxY motif-containing cargo proteins and the µ2 adaptin of AP-2 interacts with the YxxΦ motif-containing cargo proteins. Our recent data demonstrate that the DH domain may recruit cargo, such as CFTR that does not have a canonical NPxY motif (20). The Dab2 NPF repeats and the β2 adaptin of AP-2 bind clathrin. Moreover, Dab2 and AP-2 interact with each other by binding of the Dab2 DPF motif to the α adaptin of AP-2.
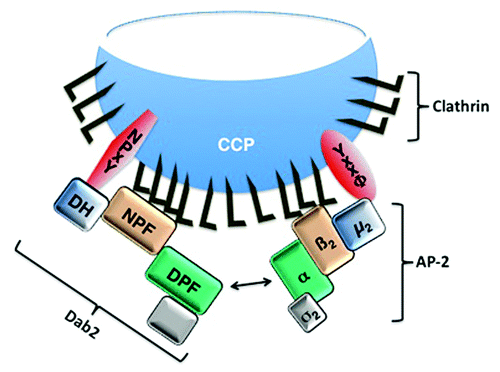
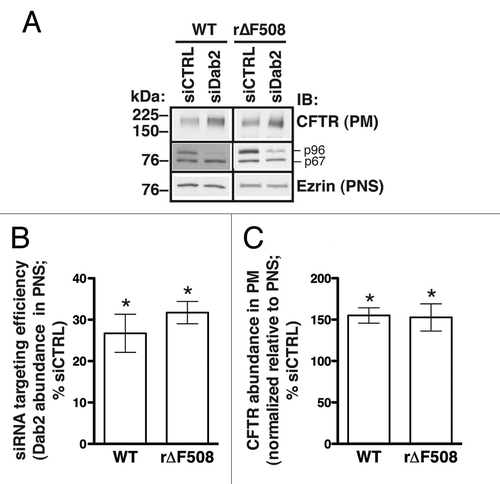
Figure 2. Experiments performed to determine the effects of Dab2 on the cell membrane abundance of WT-CFTR and r∆F508-CFTR in human airway epithelial cells (CFBE41o-). Cells were transfected with siRNA specific for the human Dab2 gene (siDab2) or with a non-silencing siRNA control (siCTRL). The Dab2 gene is alternatively spliced to produce two proteins, p96 and p67 (30). Because the Dab2 p96 functions as an endocytic adaptor, it was specifically targeted by the siRNA. CFBE41o- cells expressing ∆F508-CFTR were cultured at 27°C for 36 h before experiments to increase biosynthetic processing and thus enhance the cell membrane abundance of rescued ∆F508-CFTR (r∆F508-CFTR). Representative western blots (A, middle row) and summary of experiments (B) demonstrate that siDab2 decreased the Dab2 p96 protein abundance in postnuclear supernatants (PNS) by ~70%. Silencing Dab2 increased the steady-state plasma membrane (PM) abundance of WT-CFTR and r∆F508-CFTR (A, top row; C). PM CFTR was isolated by cell membrane biotinylation. siDab2 did not alter ezrin expression in PNS. *, p < 0.05 vs. siCTRL. Summary data reflect 4 experiments (WT-CFTR) or 6 experiments (r∆F508-CFTR). Error bars, SE.