Figures & data
Figure 1. Schematic representation of centromeric and pericentromeric chromatin and the formation of an epigenetic complex that further shapes the kinetochore. Centromeric chromatin is shaped mainly by the presence of rigid CENP-A nucleosomes, interspersed H3-CENP-T/W complex and interspersed histone H3 nucleosomes, that present the repressive marks, H3K9me2/me3 and H3K27me1/3 (“♦” indicates marks only found in plants species and “★” indicates marks reported in more than one species), active marks scattered throughout the region, H3k4me2 and acH4, and ncRNAs that form the foundation. This foundation then recruits the CCAN that will further link the other complexes that recruit the microtubules needed for chromosome segregation. In mammals, many of the CENP proteins that form the inner centromere are recruited by DNA interactions and histone mark-dependent proteins because there is evidence that Mis12C depends on HP1 for its incorporation into the kinetochore. Aurora-B/INCENP is recruited from methylated pericentromeric chromatin in order for Mis12C to interact with NDC80C (represented by the brown arrow). Aurora-B/INCENP dimer is removed from the kinetochore by phosphorylation of H3S10 Pericentromeric chromatin is mainly constitutive heterochromatin composed of repetitive satellite DNA that is heavily methylated and enriched with repressive marks, principally H3K9me3 and scattered H3K27me3, dependent on SUV39H1 and EZH1, which serve to recruit HP1, HDAC, and DNMT. Pericentromeric chromatin is further stabilized and regulated by ncRNAs generated from these satellites regions
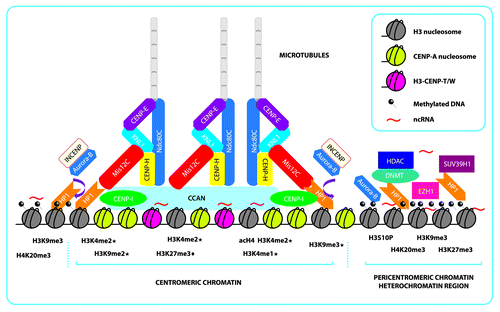
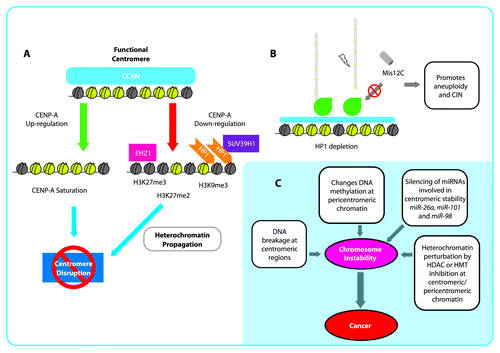
Figure 2. Epigenetic disruption of the centromere and implications for chromosomal instability (CIN). (A): Schematic representation of centromere disruption pathways by CENP-A upregulation and heterochromatization. B: HP1 depletion causes that localization of Misc12C to the kinetochore is reduced, which may promote microtubule misincorporation and kinetochore unsteadiness generating aneuploidy and CIN. C: Chromosomal instability generated by different mechanisms at centromeric and pericentromeric regions as a plausible early cause of cancer.