Figures & data
Figure 1. Origin and fate of TMA in the human gut, and the Archaebiotics concept. Gut microbiota synthesis of TMA is realized from TMAO, choline, PC and L-carnitine. The TMA is then absorbed and goes to the liver, routes (A or (B). In the case of route (A), a partial or total defect in FMO3-oxidation into TMAO leads to increased level and diffusion of TMA in breath, urine and sweat. When FMO3 liver oxidation is functional (B), the increase of TMAO in blood is associated with atherosclerosis.Citation2,Citation7,Citation11 Therefore, converting TMA directly in the gut using Archaebiotics belonging to the seventh methanogenic order, naturally-occurring in the gut, route (C) should be envisaged. Interestingly, these archaea are only able to perform methanogenesis using methyl compounds (see ), because the two other pathways are absent (CO2 reduction with H2 and aceticlastic pathway): this would increase the efficiency of TMA conversion.
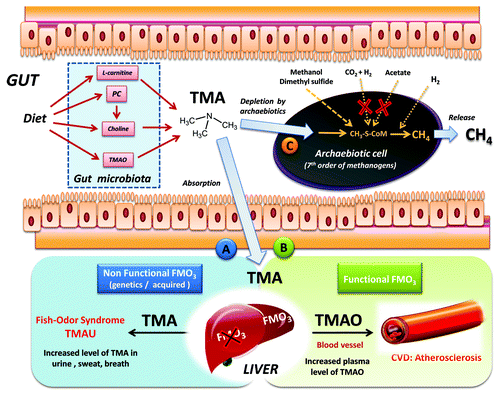
Figure 2. Metabolic pathway of TMA conversion deduced from genomic data from species of the 7th order of methanogens. The pathway of TMA conversion involves a trimethylamine:corrinoid methyltransferase (MttB) which contains a pyrrolysine, an unusual amino-acid. The synthesis of this amino-acid and its transfer on a tRNAPyl to form a pyrrolysyl-tRNAPyl, and its insertion during the translation of mttB are presented in the green part. The pathway of trimethylamine (TMA) conversion, involving the pyrrolysine containing MttB, is presented in the orange part. Steps in the pathway between named compounds are indicated by blue arrows. The purple arrow indicates the transcription and translation of mttB. The dotted arrows indicate steps that are not detailed for the clarity of the scheme. Recognized enzyme names (see KEGG map 00680) are provided, flanked by the corresponding protein accession numbers in the “Ca. M. alvus” (blue) and “Ca. M. intestinalis” (red) genomes. Only accession numbers of enzymes specifically involved in the depletion of TMA are presented, some being shared with dimethylamine (DMA) and monomethylamine (MMA) depletion (not shown here).
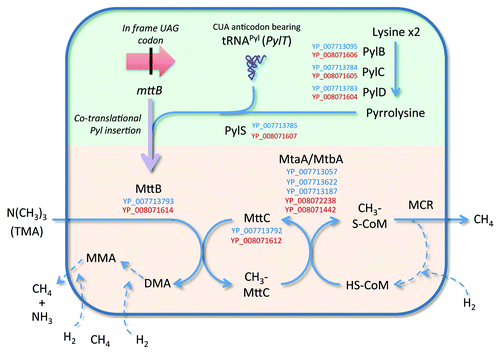
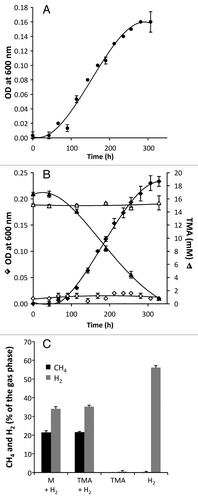
Figure 3. Growth of Methanomassiliicoccus luminyensis strain B10 on trimethylamine (TMA) and hydrogen (H2). (A) The growth of M. luminyensis B10 on methanol (M, 50 mM) and H2 (previously described by Dridi et al.Citation18) was used as a positive control for growth and maximal cell density. (B) We now show that M. luminyensis B10 also grows on TMA (15 mM) in the presence of H2 (filled diamonds) but not on TMA in the absence of H2 (open diamonds). Accordingly, TMA is depleted in presence of H2 (filled triangles) and not in the absence of H2 (open triangles). The composition of the gas phase was measured at the end of the experiment (C) and revealed the production of methane (CH4) and the depletion of H2 in presence of either M or TMA. No CH4 was produced in the absence of H2 and CH4 was not produced nor H2 depleted in the absence of the methylated compound substrate (M or TMA). M. luminyensis B10 was obtained from DSMZ (DSM No. 25720) and cultivated in DSMZ medium 119, with rumen fluid replacing the sludge fluid. When H2 was present, initial atmosphere composition was: N2/H2/CO2 (55:35:10), and in absence of H2: N2/CO2 (75:25). Growth in each condition was performed in triplicate. Trimethylamine concentration was measured as described by Krätzer et al.Citation30 OD, Optical Density.