Figures & data
Figure 1. Chronological order of experiments for the identification of Flu-specific clones for BC-143. (A) T-cell responses measured by IFN-γ ELISPOT assay: CD8+ T-cell responses to Flu-M- and SSX-2-peptides (left) as well as CD4+ T-cell response to Flu-NP- and SSX-2-peptides (right). Five × 104 (upper part) and 1 × 104 (lower part) cells per well were used. (B) T-cell responses measured by IFN-γ-secretion assay: CD4+ T-cell responses to Flu-NP and SSX-2 peptides by IFN-γ secretion (upper part) and CD8+ T-cell responses to Flu-M- and SSX-2-peptides measured by a combination of IFN-γ secretion and tetramer staining (lower part). (C) Clone specificity analysis: Analysis of CD8+ T-cell clone specificity via IFN-γ ELISPOT (upper part) and tetramer-FACS analysis (lower part).
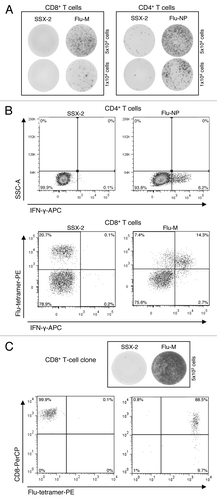
Figure 2. Schematic representation of vector constructs used in this study. The lentiviral vector (A) “Lego-MP-muFlu” and the gammaretroviral vector (B) “MP91-muFlu” are displayed [not to scale]. MPSV, myeloproliferative sarcoma virus; LTR, long-terminal repeat; MESV, murine embryonic stem cell virus; IRES, internal ribosome entry site; wPRE, Woodchuck hepatitis virus post-transcriptional regulatory element; P2A, 2A sequence of porcine teschovirus-1; eGFP, enhanced green fluorescent protein; SD, splice donor; SA, splice acceptor.
![Figure 2. Schematic representation of vector constructs used in this study. The lentiviral vector (A) “Lego-MP-muFlu” and the gammaretroviral vector (B) “MP91-muFlu” are displayed [not to scale]. MPSV, myeloproliferative sarcoma virus; LTR, long-terminal repeat; MESV, murine embryonic stem cell virus; IRES, internal ribosome entry site; wPRE, Woodchuck hepatitis virus post-transcriptional regulatory element; P2A, 2A sequence of porcine teschovirus-1; eGFP, enhanced green fluorescent protein; SD, splice donor; SA, splice acceptor.](/cms/asset/a9c5c68f-cdb5-4423-a268-b63852200be5/khvi_a_10924051_f0002.gif)
Figure 3. Expression of the muFlu-vector constructs in J76 cells. J76 cells were transduced with LeGO-MP-muFlu, MP91-muFlu and LeGO-iG2, serving as a mock control. (A) 3 d post transduction eGFP-, CD3- and mTCR- expression were analyzed. Numbers indicate percentages of eGFP, mTCR and CD3 positive cells, respectively. (B) eGFP positive cells were double-stained with the mTCR- and CD3-specific antibodies. eGFP+ cells expressing both CD3 and the Flu-TCR are depicted in the upper right quadrant.
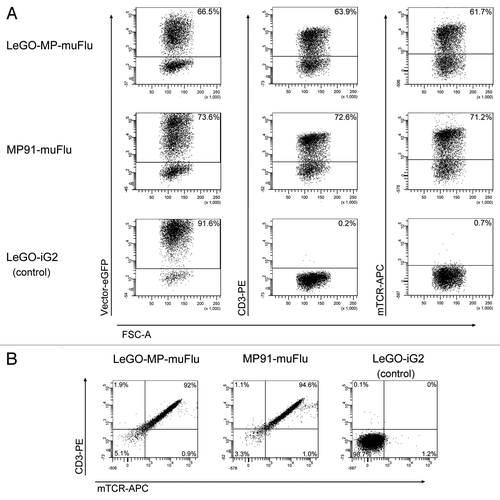
Figure 4. Specific IL-2 secretion of Flu-transduced J76 cells upon encounter with the Flu-M-peptide. J76 cells were stably transduced with the lentiviral construct LeGO-MP-muFlu and the gammaretroviral construct MP91-muFlu or mock-transduced with LeGO-iG2. Transduced cells were co-cultured with Flu- or SSX-2- peptide pulsed T2 cells, serving as APCs. After 18–20 h the supernatant was harvested. IL-2 secretion was analyzed by ELISA. Mean values and range of two independent experiments are shown. No IL-2 secretion was detectable for J76 cells stimulated with the irrelevant peptide SSX-2 and for mock-transduced J76 cells. n.d., not detectable.
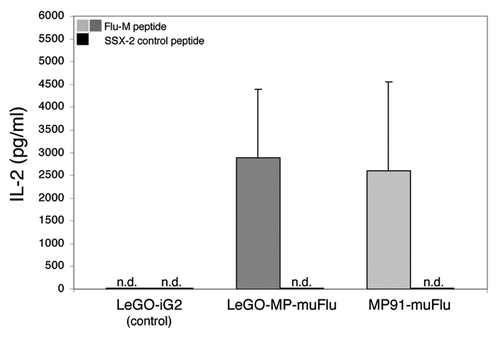
Figure 5. Transduction of peripheral blood T cells with the LeGO-MP-muFlu-vector construct. Peripheral blood T cells of buffy coat BCBBe3 were transduced with LeGO-MP-muFlu 72 h after activation with CD3/CD28-Dynabeads. Transduction efficiencies were measured 3 d post transduction via FACS, looking at eGFP-, mTCR- and Flu-tetramer-expression and staining, respectively. Numbers indicate percentages of eGFP, mTCR and Flu-tetramer positive cells.
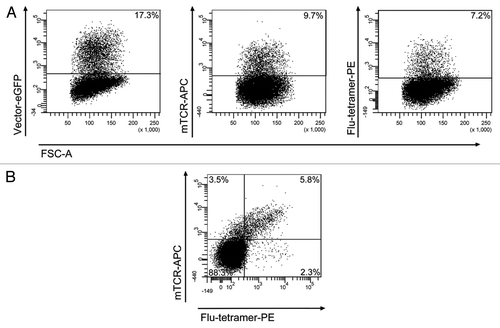
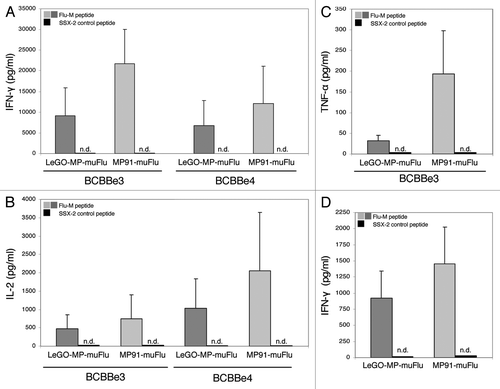
Figure 6. IL-2-, IFN-γ and TNF-α- secretion by transduced primary human T lymphocytes upon co-culture with the Flu-M vs. SSX-2- peptide. Primary T cells were transduced with LeGO-MP-muFlu or MP91-muFlu 72h after activation of PBMCs with CD3/CD28-Dynabeads. Five days after transduction cells were co-cultured with peptide (Flu-M, SSX-2) pulsed T2 cells and the secretion of different cytokines was analyzed by ELISA. (A–C) Mean values and ranges for two to three independent experiments with two buffy coats (BCBBe3, 4) are shown. (A) IFN-γ, (B) IL-2, (C) TNF-α. (D) IFN-γ cytokine secretion of transduced primary cells showing the same amount of Flu-tetramer positive cells were compared by ELISA. Mean values of 3 different buffy coats and two experiments are shown. n.d., not detectable.