Figures & data
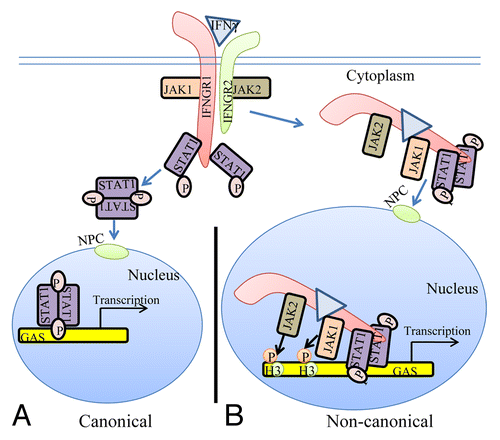
Figure 1. The classical and non-canonical models of IFNγ signaling. (A) In the classical model of IFNγ signaling, IFNγ crosslinks the IFNGR1 receptor subunit that results in allosteric changes in receptor cytoplasmic domain causing the movement of JAK2 from receptor subunit IFNGR2 to IFNGR1. The JAKs autophosphorylate and then phosphorylate IFNGR1 cytoplasmic domain. This results in binding, phosphorylation, and dimer formation of STAT1α. The dimeric STAT1α dissociates from receptor and undergoes nuclear translocation via an intrinsic NLS for specific gene activation. (B) The non-canonical model of IFNγ signaling involves IFNγ binding to receptor extracellular domain, followed by movement to IFNGR1 cytoplasmic domain in conjunction with endocytosis. The cytoplasmic binding increases the affinity of JAK2 for IFNGR1, which is the basis for its movement to IFNGR1. This results in autoactivation of the JAKs, phosphorylation of IFNGR1 cytoplasmic domain, and the binding and phosphorylation of STAT1α at IFNGR1. The complex of IFNGR1/STAT1α/JAK1/JAK2 undergoes active nuclear transport where the classic polycationic NLS of IFNγ plays a key role for this transport to genes in the nucleus that are specifically activated by IFNγ. Furthermore, the JAKs associated with the specific promoters were shown to be involved in epigenetic modifications. Details of the non-canonical model are presented in the text. GAS, IFN gamma activated sequence; H3, histone H3; NPC, nuclear pore complex.