Figures & data
Figure 1 Key immune system interactions are targeted by approved therapeutic mAbs. This figure illustrates the immunological pathways targeted by the approved mAbs and Fc-fusion proteins summarized in Table 1. CD, cluster of differentiation; CTLA-4, cytotoxic T-lymphocyte antigen-4; EpCAM, epithelial cell adhesion molecule; GM-CSF, granulocyte macrophage-colony stimulating factor; HLA, human leukocyte antigen; ICAM, intercellular adhesion molecule; IFN, interferon; Ig, immunoglobulin; IL, interleukin; LFA, lymphocyte function-associated antigen; TNF, tumor necrosis factor; LT, lymphotoxin; RANKL, receptor activator of nuclear factor kappaB ligand; TH cell, T helper cell; TRAIL, TNF-related apoptosis-inducing ligand; VCAM, vascular cell adhesion molecule; VLA, very late antigen.
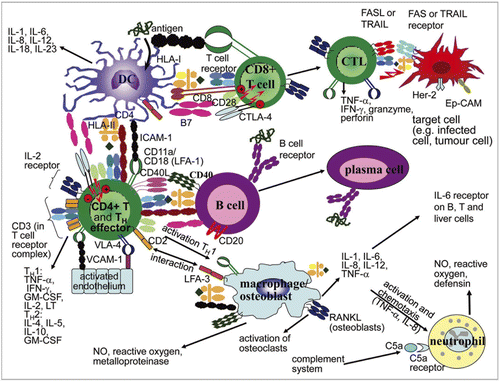
Figure 2 Strategy and timing for assessment of immunotoxicity with immunomodulatory mAbs. A range of immunological tests can be applied at different stages of the product development program of a mAb. *May be required depending on the MoA of the mAb and/or to investigate the mechanism of any immune function defects observed in non-clinical or clinical studies.
Figure 3 T cell-dependent and -independent induction of anti-drug antibody formation. In most cases, formation of anti-drug antibodies is T cell-dependent (A). T cell activation requires preceding activation of professional APCs such as DCs. Immature DCs (im DC) scan their direct environment constantly for danger signals, while they ingest the surrounding matrix by fluid phase or receptor mediated endocytosis. Ligation of pattern recognition receptors (PRRs) by danger-associated molecular patterns (DAMPs) such as exposed hydrophobic structures of aggregated proteins or pathogen-associated molecular patterns (PAMPs) such as LPS and ligation of Fcγ receptors (FcγR) induce immature DCs to mature and migrate to draining lymph nodes. Drugs that were taken up by the immature DCs will then get processed to small peptides. If these peptides have the appropriate primary structure, they form a complex with HLA class II molecules (HLA II:peptide). In the lymph node, the mature DCs (mat DC) will present the HLA II:peptide complexes to T cells, while also providing the additional signals required to prime naïve CD4+ T cells (co-stimulatory molecules such as C80 and CD86, as well as cytokines such as IL-10 and IL-4). Naïve B cells expressing a B cell receptor that recognize the drug will specifically endocytose the drug and process it to the same peptides, which are generated by the DCs. This allows the activated drug specific CD4+ T cells to induce drug specific B cells to proliferate and differentiate into memory B cells and plasma cells. The same cellular activation steps are required to react to neo antigens derived from non-human or modified protein and to break down tolerance to drugs with fully human protein sequences. In contrast, aggregated drug may induce B cell activation directly by cross-linking of the drug or drug aggregate specific B cell receptor on the surface of B cells (B). However, in this case typically no isotype switching or memory formation is observed. As aggregated drug can also induce the T cell-dependent B cell activation by affecting the DC activation, the two pathways may also be synergistic in some cases. The presence of DAMPs and PAMPs in drug substance or drug product can be investigated in vitro by using monocyte-derived DCs. In silico immunogenicity prediction tools focus on the capability of a defined protein sequence to bind to HLA class II molecules. A more reliable approach to identify drug-derived peptides which are presented on APCs is to sequence the peptides which were eluted from HLA class II molecules expressed by monocyte-derived DCs after challenge with the protein drug. Furthermore, in vitro T cell activation assays allow investigation of the whole process from antigen uptake, through antigen processing, to the capability of the generated peptides to activate naïve T cells. As there is currently no in vitro tool available to investigate T cell-dependent or -independent B cell activation, the whole process can only be investigated by using transgenic or double transgenic mouse models, with the drawback that the immune system of mice and humans appears to be quite different.
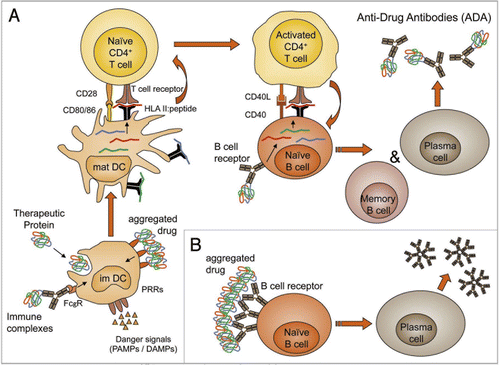
Table 1 FDA and/or EMA-Approved mABs and Fc-fusion proteins
Table 2 Key guidelines relevant to non-clinical safety testing of mAbs
Table 3 Key functional characteristics of human IgG subclasses