Figures & data
Table 1. Overview of (potential) advantages of bispecific heterodimeric antibodies over classical IgG antibodies and their combination
Figure 1. (A) Schematic depiction of the homo- and heterodimerization interfaces between light- and heavy-chain domains leading to mixtures when expressed simultaneously. (B) Chain association issue when co-expressing two different antibody heavy and light chains in one cell line, assuming random chain association (Quadroma). In total, 24 = 16 combinations are possible. Of those, 6 are identical; thus, a purely statistical association leads to 6 tetramers that occur twice (each 12.5% yield) and 4 tetramers that occur once (each 6.25%). The desired bispecific antibody makes up statistically 12.5% of the total yield. (C) Light chain association issue when co-expressing two different antibody light chains in one cell line, assuming random chain association. Heavy chain heterodimerization is enforced using KiH technology. The desired bispecific antibody makes up statistically 25% of the total yield.
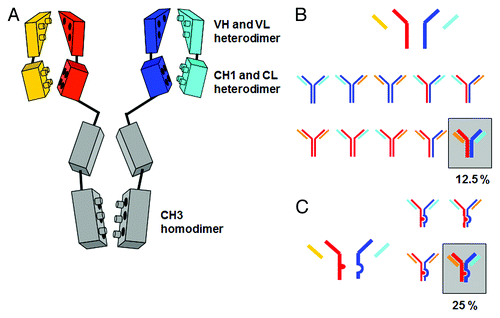
Figure 2. Overview of bispecific heterodimeric IgG antibodies with heterodimeric Fc-region. (A) Quadroma approach with isolation of the desired bispecific Triomab. (B) KiH approach with two different light chains based on in vitro assembly; the white circle indicates the lack of glycosylation due to expression in E. coli. (C) KiH approach with common light chain. (D) CrossMabCH1-CL based on KiH approach in combination with light chain crossover. (E) (SEED)body approach based on strand exchange between IgG and IgA CH3 domains. (F) LUZ-Y with C-terminal fusion of a leucine zipper to the heavy chain to ensure HC heterodimerization and common light chain. The leucine zipper can subsequently be cleaved off proteolytically. For a more detailed description, refer to the text.
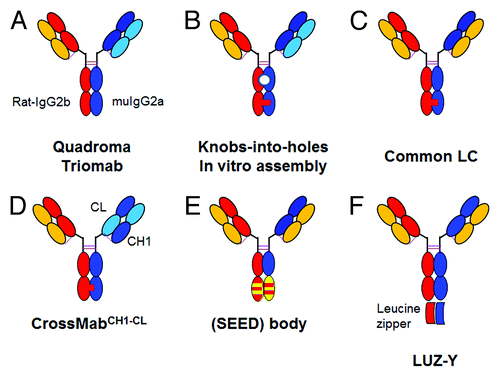
Figure 3. Enforcing correct heavy chain association by CH3-CH3 interface modification. Structural model of heterodimeric Fc (one CH3 domain as black line, the other as gray line) with (A) KiH mutations and S-S stabilization (1 knob mutation, blue; 3 hole mutations, red; one disulfide bridge, yellow).Citation25 (B) charged residues located at the CH3-CH3 interface (Glu, Asp, red; Lys blue) that can typically be used to enforce heterodimerization by appropriate exchange as shown by Amgen and Chugai. (C) optimal variant (4 mutations, purple and magenta) obtained by Xencor. (D) schematic representation of (from left) the desired heterodimer and the two unwanted homodimers obtained by the KiH method (top) or use of electrostatic steering (bottom).
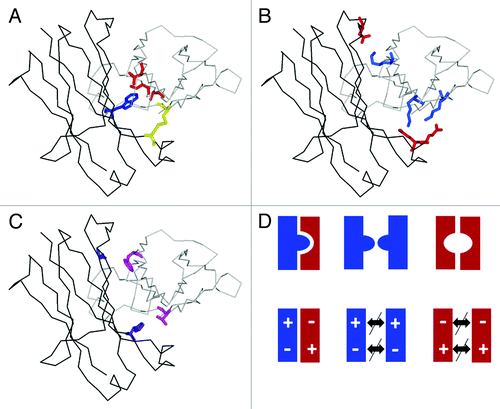
Figure 4. CrossMab principle. Starting from a conventional IgG antibody correct chain association in a bispecific heterodimeric antibody can be achieved using the KiH technology to enforce correct heavy chain heterodimerization in combination with domain crossover of light chain domains to enforce correct light chain association. The three possible light chain domain crossovers are depicted: Fab domain crossover on the left, VH-VL domain crossover on the right and CH1-CL crossover on the top.
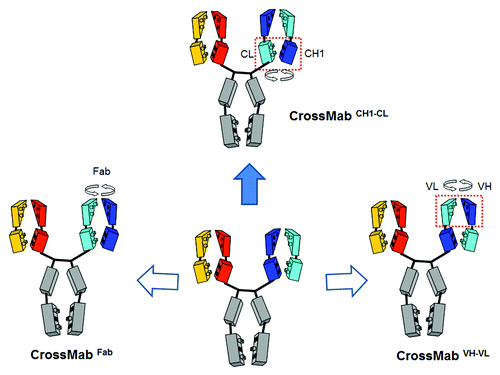
Figure 5. Overview of bispecific heterodimeric IgG antibodies. (A) Common light chain bispecific antibody with H435R Y436F mutation to allow depletion of hole-hole antibodies. (B) Duobody approach based on IgG4 Fab arm exchange with in vitro assembly. (C) Bispecific heterodimeric IgG1/IgG2 with EEE/RRR mutations with in vitro assembly. (D) Dual-acting Fab (DAF) IgG or pan-specific monoclonal antibodies. (E) MabCitation2 approach with introduction of additional binding sites in the Fc region of an IgG antibody. (F) CovX body approach with fusion of a bispecific binding peptide into the active center of a catalytically active IgG antibody. The bispecific binding peptide is indicated on the top. For a more detailed description, refer to the text.
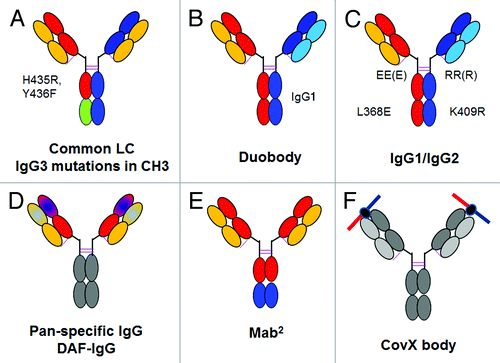
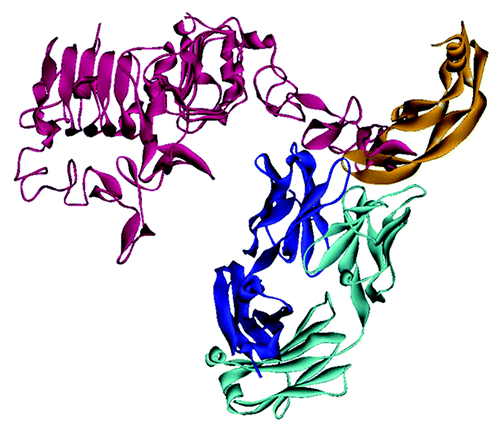
Figure 6. HER2- and VEGF-binding dual acting antibody (DAF)Citation62,Citation63 shown as superposition of the complex structures with HER2 (PDB:3bdy) and with VEGF-A (PDB:3be1). The picture illustrates that HER2 (red) interacts mainly with the heavy chain of the antibody (dark blue), whereas VEGF-A (orange) interacts almost exclusively with the light chain (light blue). There are no significant interactions within the unrelated pairs HC-VEGF and LC-HER2. The Fab of 3be1 has been omitted for clarity since both Fabs exhibit an almost identical structure.