Figures & data
Table 1. Results of testing the swirling protocol with Remicade® and Herceptin®
Figure 3. UV-Vis absorbance (A), 90° light-scattering (B), intrinsic fluorescence emission (C) and Nile red fluorescence microscopy as particles/ml (D) of Herceptin® and Remicade® solubilized using different procedures. Black, recommended manual procedure (I); blue, robotic procedure (II); red, recommended manual procedure with strong shaking (III); gray, placebo solution.
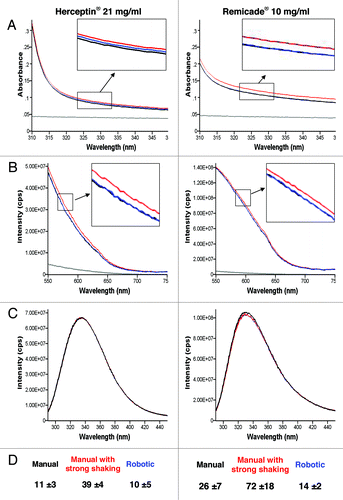
Figure 4. Effect of the solubilization procedure on the molar mass vs. time graphs with UV signals at 280 nm (solid lines) of Herceptin® (1.06 mg/ml mAb in 0.9% sodium chloride) and Remicade® (1.23 mg/ml mAb in 0.9% sodium chloride) with 0.9% sodium chloride in water and 200 ppm NaN3 as running buffer. Ten μl of each protein sample were injected three times. The antibody solutions were diluted with 0.9% sodium chloride just before the FFF measurements. Black: recommended manual procedure (I); blue: robotic procedure (II) and red: recommended manual procedure with strong shaking (III).
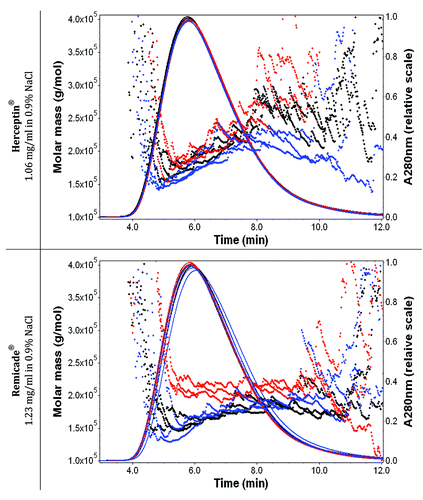
Figure 5. UV-Vis absorbance (A), 90° light-scattering (B), intrinsic fluorescence emission (C), and Nile red fluorescence microscopy as particles/ml (D) of Herceptin®, Remicade® and Avastin® solutions (samples solubilized as the recommended procedure) and after stress of the solution by aspiration-dispense through a 18G 2” 1.2 × 50 mm needle. Black, Time 0; blue, after 1× aspiration/dispense; green, after 5× aspiration/dispense; red, after 15× aspiration/dispense.
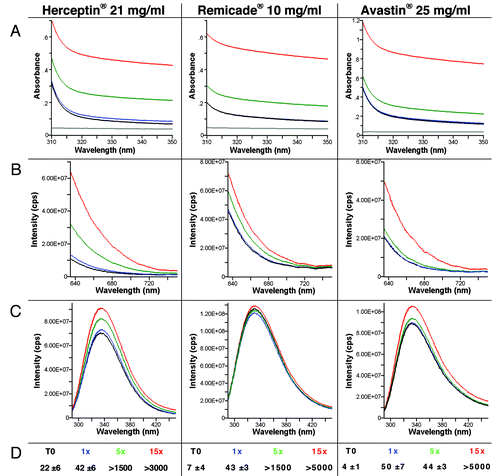
Figure 6. Effect of aspiration-dispense cycles on the molar mass (linear scale) vs. time graphs with UV signals at 280 nm (solid lines) of Herceptin® (1.06 mg/ml mAb in 0.9% sodium chloride), Remicade® (1.23 mg/ml mAb in 0.9% sodium chloride) and Avastin® (1.33 mg/ml mAb in 0.9% sodium chloride) with 0.9% sodium chloride in water and 200 ppm NaN3 as running buffer. Ten μl of each protein sample were injected once. The antibody solutions were stressed and then diluted with 0.9% sodium chloride just before the FFF measurements. Black, Time 0; blue, after 1× aspiration/dispense; green, after 5× aspiration/dispense; red, after 15× aspiration/dispense.
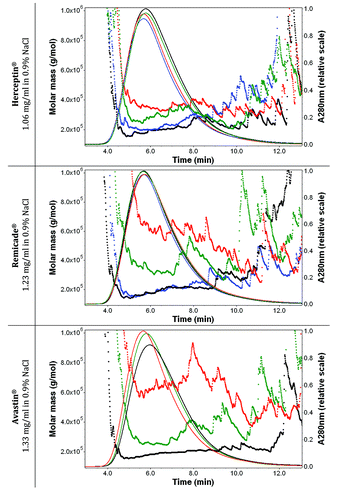
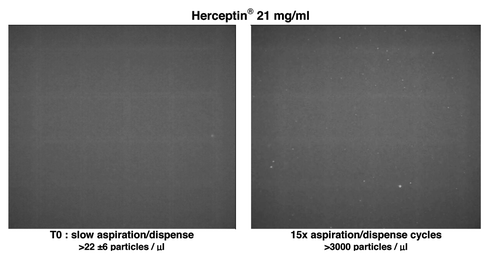
Figure 7. Effect of aspiration dispense cycles. Nile red fluorescence microscopy of Herceptin® solution (sample solubilized as the recommended procedure) before and after stress of the solution by aspiration-dispense through a 18G 2” 1.2 × 50 mm needle.