Figures & data
Figure 1.CD28 expression by human, baboon and cynomolgus macaque memory T cells. One representative immunophenotyping analysis of CD28 in CD4+ (upper panel)and CD8+ (lower panel) memory (CD95+) and naïve (CD95-) T lymphocytes of human, baboon and cynomolgus macaque PBMC. Data are representative of at least three independent analyses.
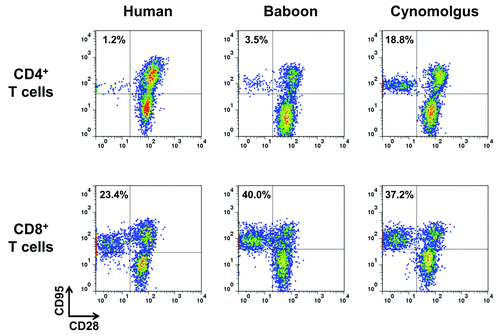
Figure 2.Proliferation and cytokine release of human PBMC co-cultured with human endothelial cells in response to anti-CD28 mAbs. (A) Proliferation at 72 h, (B) IFNγ, (C) TNF,(D) IL-2, (E) IL-4, (F) IL-5, (G) IL-6 and (H) IL-12p70 release at 48 h after addition of 0.4, 2, 10 or 50 µg/ml of humanized pegylated Fab’ fragment FR104, divalent agonist anti-CD28.2 mAb and superagonist anti-CD28 ANC28.1 mAb or of an equivalent volume of excipient. Histogram bars are means ± SEM from data obtained with four different blood donors. LLOQ: Lower limit of quantification.
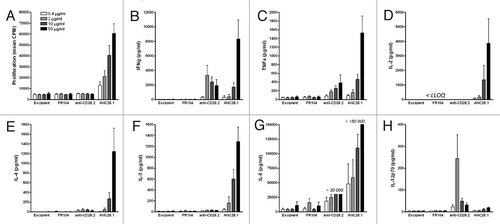
Figure 3.Proliferation and cytokine release of baboon PBMC co-cultured with human endothelial cells in response to anti-CD28 mAbs. (A) Proliferation at 72 h, (B) IFNγ, (C) TNF, (D) IL-2, (E) IL-6 and (F) IL-12/23p40 release at either 24 and 48 h after addition of excipient (0),0.5, 5 or 50 µg/ml of divalent agonist anti-CD28.2 and superagonist anti-CD28 ANC28.1 mAbs. Data are mean ± SEM of 4 different blood donors.
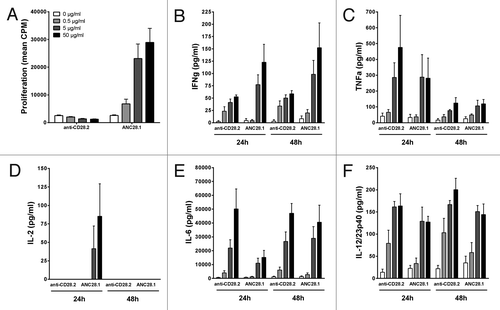
Figure 4.In vivo stimulation of human or baboon PBMC with anti-CD28 mAbs in NSG mice. One representative flow cytometry analysis (upper panel) and quantification of engraftment (low panel) of (A) human (n = 20) and (B) baboon (n = 18) cells in NSG mice two weeks after PBMC transfer. Each mark represents an individual mouse. (C, D) Percentage of activated human and baboon T lymphocytes expressing CD25 and CD69 among CD3+ cells in recipient blood and spleen 48 h after drug injection. Two weeks after PBMC transfer, mice received either 50 µg i.p. of superagonist anti-CD28 ANC28.1 mAb (red square symbols; n = 5 for human and n = 7 for baboon), 150 µg of humanized pegylated Fab’ fragment FR104 (blue dots; n = 6 for human and n = 5 for baboon) or an equivalent volume of excipient (open diamond; n = 5 for human and baboon). Each symbol represents an individual mouse. Horizontal bars indicate the mean. (E) Human and (F) baboon cytokine release after treatment in same experiments as in C and D; data are means ± SEM.*P < 0.05 and **P < 0.01 compared with control conditions. Cumulative data were obtained from three independent series of experiments using three different human and three baboon blood donors.
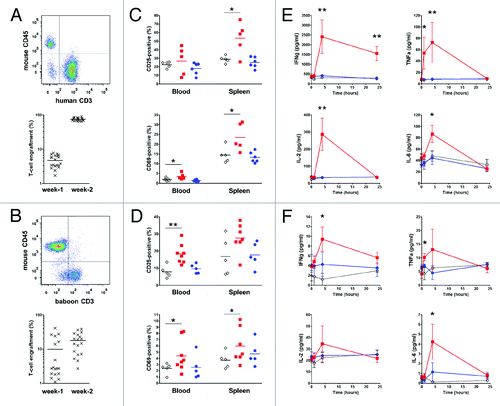
Figure 5.Immunotoxicity evaluation of FR104 after intravenous injection in baboon. (A) Serum concentration (black square; left y-axis) of FR104 and receptor occupancy (open square; right y-axis) on blood T lymphocytes measured by flow cytometry after a single intravenous administration at 20 mg/kg in three different baboons. Data are mean ± SEM (B) Serum concentration of indicated cytokines measured after FR104 injection in the three animals described in (A). Data are mean ± SEM. Dotted line represents the highest lower limit of quantification (LLOQ). (C) leukocytes count, (D) lymphocytes count, (E) platelets count, (F) hemoglobin count, (G) body temperature, (H) cardiac frequency, (I) mean arterial pressure and(J) oxygen saturation recorded after FR104 injection in the three animals described in (A; black symbols and solid lines) as well as in two other excipient-treated baboons (open symbols and dotted lines). Each animal is represented by a different symbol. The horizontal lines indicate the range of normal values in baboons.Citation39
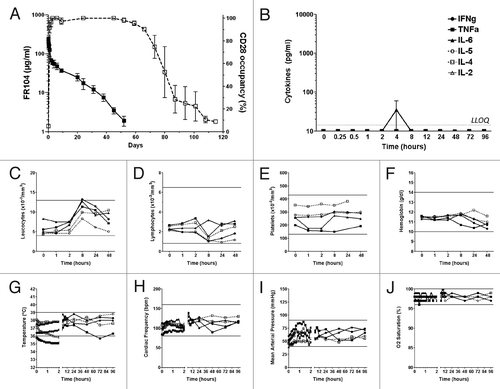
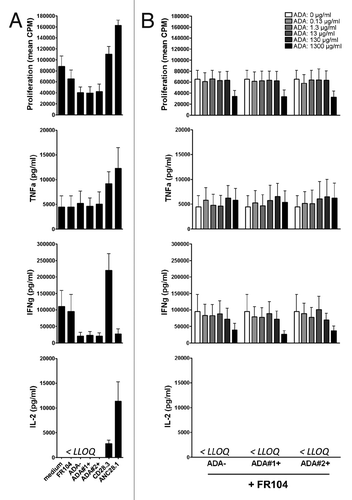
Figure 6.In vitro response of human PBMC to antagonist anti-CD28 mAbs and ADA. Human PBMC were stimulated with anti-CD3 antibodies and different types of anti-CD28 mAbs plus indicated concentrations of IgG containing ADA. (A) Proliferation at 72 h and cytokine release at 48 h in the presence of excipient, FR104 (10µg/ml), divalent agonist anti-CD28.3 mAbs (10 µg/ml), superagonist anti-CD28 ANC28.1 mAbs (10µg/ml), ADA-negative purified IgG (1.3 mg/ml), or ADA-positive purified IgG from two different baboons (1.3 mg/ml).(B) Same experiments as in (A) where human PBMC were cultured with 10 µg/ml of FR104 and increasing concentrations of ADA-negative or ADA-positive purified IgG (ranging from 0 to 1300 µg/ml as indicated in the upper panel).Data are means ± SEM of 3 different human blood donors. LLOQ: Lower limit of quantification.