Figures & data
Figure 1. Effect of itolizumab treatment on T cell proliferation capacity in psoriasis patients. PBMC were stimulated with anti-CD2/CD3/CD28 beads for 96 h and precursor frequency (PF) was calculated to determine the percentage of dividing cells in each patient at baseline (BL), after induction phase (AI), during maintenance phase (DM) and after maintenance phase (AM). Healthy donor (HD) PBMC were used as control. Individual values and means ± SD are represented. (A) Patients who received 0.4 mg/kg in the induction phase and 1.6 mg/kg in the maintenance phase. (B) Patients who received 1.6 mg/kg in both phases. Wilcoxon Signed Ranks Test was used to compare patient’s samples; Mann Whitney U Test was used to compare patients with HD; *P < 0.05, **P < 0.01; ns: non-significant.
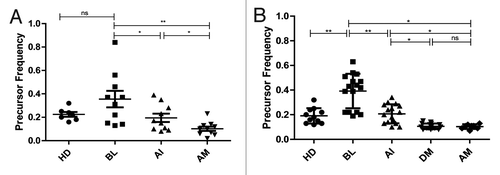
Figure 2. Influence of itolizumab treatment in the number of IFN-γ-secreting PBMC from psoriasis patients. PBMC were stimulated with a human anti-CD3 antibody and IFN-γ release in response to stimulation was tested by ELISpot. Numbers of spots-forming units (SFU) were counted at baseline (BL), after induction phase (AI) and after maintenance phase (AM). Healthy donor (HD) PBMC were used as control. Individual values and means ± SD are represented. Wilcoxon Signed Ranks Test was used to compare patient’s samples; Mann Whitney U Test was used to compare patients with HD; *P < 0.05; ns: non-significant.
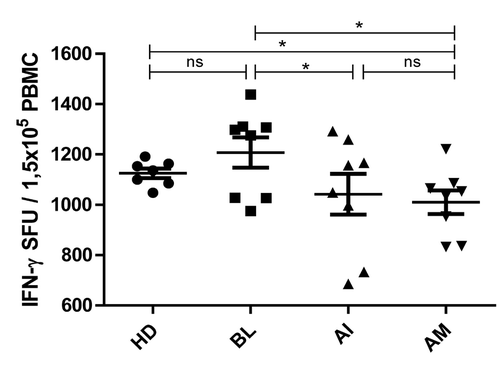
Figure 3. Cytokine profile during itolizumab treatment of psoriasis patients. Serum concentration of cytokines 3.1: IL-6 (pg/ml), 3.2: IL-8 (pg/ml), 3.3: IL-10 (pg/ml), 3.4: TNF (pg/ml) and 3.5: IFN-γ (pg/ml) were evaluated at baseline (BL), after induction phase (AI), during maintenance phase (DM) and after maintenance phase (AM), by ELISA. Healthy donor (HD) sera were used as control. Individual values and means ± SD are represented. (A) Patients who received 0.4 mg/kg in the induction phase and 1.6 mg/kg in the maintenance phase. (B) Patients who received 1.6 mg/kg in both phases. Wilcoxon Signed Ranks Test was used to compare patient’s samples; Mann Whitney U Test was used to compare patients with HD; *P < 0.05, **P < 0.01; ns: non-significant.
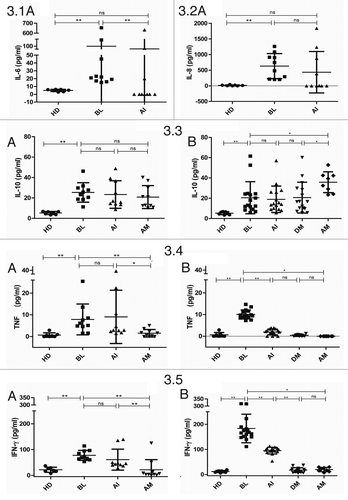
Figure 4. Effect of itolizumab treatment in the evolution of psoriatic lesions. 4.1: Histopathological study of a representative skin biopsy from a patient at baseline (BL) and after induction phase (AI). Epidermal hyperplasia was evaluated after staining with hematoxilin/eosin (magnification 25X). 4.2: Morphometric study of epidermis thickness in skin biopsies from psoriasis patients treated with itolizumab at BL and AI. Images were processed by the computer system DIGIPAT IBM/PCCitation84 and epidermis thickness was calculated as the mean of three points from each image. Individual values are represented. Wilcoxon Signed Rank Test was used; ns: non-significant.
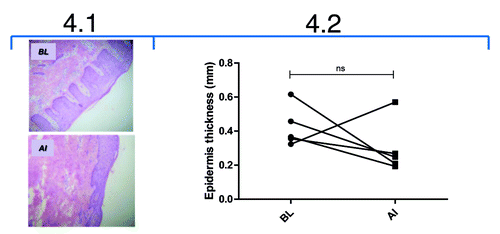
Figure 5. Immunohistochemical study of skin biopsies from psoriasis patients treated with itolizumab. 5.1: Immunohistochemical staining of infiltrating CD6+ T lymphocytes (magnification 400X) from one representative patient at baseline (BL) and after induction phase (AI). 5.2: Quantification of infiltrating CD3+ and CD6+ T lymphocytes. Individual values are represented. Wilcoxon Signed Ranks Test was used; ns: non-significant.
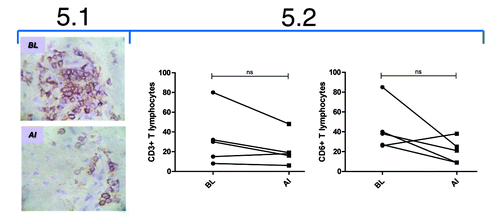
Figure 6. Correlation between the reduction indexes of infiltrating T CD3+ lymphocytes and epidermis thickness, after induction phase of itolizumab treatment of five psoriasis patients (P = 0.041, Spearman r = 0.9).
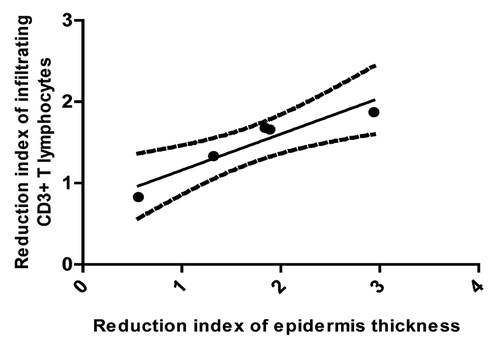
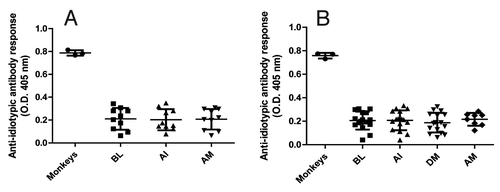
Figure 7. Anti-idiotypic antibody response to itolizumab in treated psoriasis patients. The presence of anti-idiotypic antibodies in sera from patients at baseline (BL), after induction phase (AI), during maintenance phase (DM) and after maintenance phase (AM) was determined by ELISA. Plates were coated with itolizumab F(ab´)2 and sera were assayed at 1:100 dilution. Monkey seraCitation25 were used as positive control. Individual values and means ± SD are represented. (A) Patients who received 0.4 mg/kg in the induction phase and 1.6 mg/kg in the maintenance phase. (B) Patients who received 1.6 mg/kg in both phases.