Figures & data
Figure 1. Mutagenesis mapping of hu19C11 CDRs. To measure the relative antigen binding affinity of hu19C11 Fab variants, binding of serially diluted phage displaying anti-IL4 hu19C11 wild type (wt) or LC CDR alanine mutants to IL4 (A) or to anti-gD antibody (B) coated on ELISA wells was detected by an anti-M13 phage horseradish peroxidase (HRP) conjugate to measure antigen binding and Fab expression, respectively. Single letter codes of amino acids are used. gD is an expression peptide tag fused to the C terminus of the light chain. Anti-gD antibody was directly coated on ELISA wells, whereas IL4 was captured with a non-blocking anti-IL4 antibody coated on ELISA wells. (C) Effects of alanine mutations at individual sites of CDRs were examined by comparing the relative IL4 binding affinity of phage displaying Fab variants by performing assays as (A) and (B). Relative IL4 binding affinity was determined by first fitting the data with a linear regression model then dividing the slope of IL4 binding (vs. phage concentration) with the slope of Fab expression. Low values (below the dotted line) were deemed low IL4 binding affinity relative to hu1911wt indicating disruptive mutations.
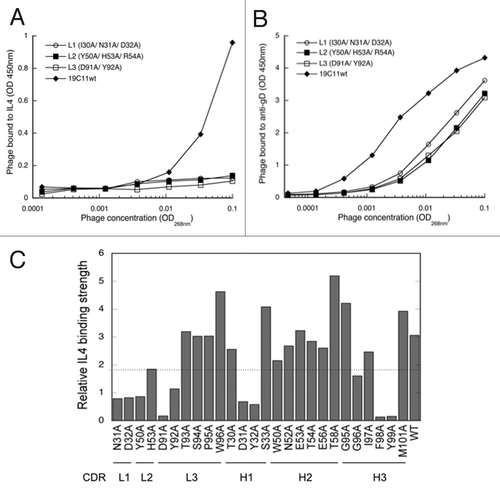
Figure 2. Dual specific clones isolated from phage libraries of h19C11 variants. (A) The structural model of hu19C11 was generated with MOE using the PDB entries 3SQO and 3BE1 as templates for the heavy and light chain, respectively. Residues important for IL4 binding, LC: 31, 32, 50, 53, 91, 92 and HC: 31, 32, 96, 98, 99 (in Kabat numbering), are colored in red on the model structure in a top down view of the antigen-binding site. Libraries of hu19C11 Fab variants displayed on phage were generated by site directed mutation. HC residues that were allowed all 20 amino acids with preference for wild type residues are labeled in bold while other HC and LC residues as labeled were allowed limited mutation to mimic natural diversity. (B) CDR sequences of selected clones including anti-IL5 specific (5A), anti-IL4/5 specific (E7, B1) and anti-IL4/13 specific (F1, F2) are shown with mutations from the parent hu19C11wt. To show the change of property upon mutation, sites included in the randomization scheme were colored according to their properties: Y, W, F in purple, hydrophobic L, I, V, A, M in gray, basic K, R, H in blue, acidic D, E in pink, polar S, T, N, Q in green, and P, G in yellow. The relative antigen binding affinity as measured by IC50 of phage competition assays are shown. Fab displaying phage was first incubated with serial dilutions of the respective antigens in solution for 2 h, unbound phage was then briefly captured by antigen coated ELISA wells and detected with anti-M13 HRP conjugate. The concentration of antigen inhibiting 50% of phage binding to antigen coated well is calculated as IC50. NB denotes no detectable binding signal of phage clone to the antigen coated wells.
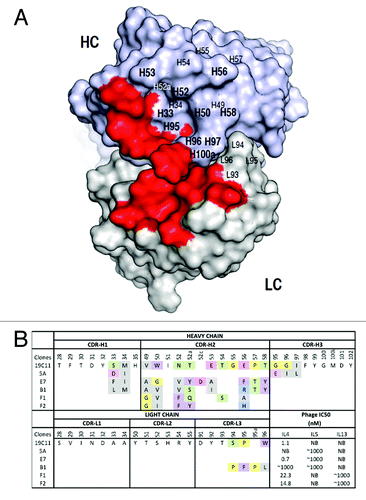
Figure 3. Dual specificity of the selected variants of hu19C11 as IgG. Antigen binding specificity of selected variants of hu19C11 was assessed as binding of these variants in human IgG1 format at 250nM to target antigen(s) or several irrelevant proteins coated on ELISA wells with detection by anti-Fc antibody HRP conjugates.
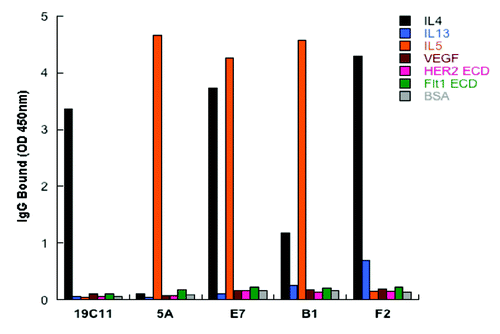
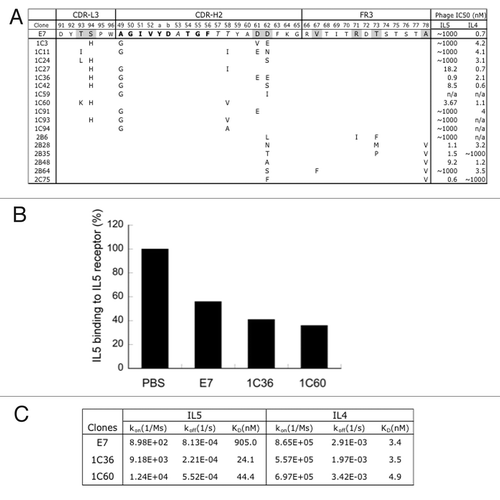
Figure 4. Affinity improvements of IL4/IL5 dual specific clone E7. (A) For affinity improvement, phage libraries displaying E7 variants were constructed with selected residues mutated with the strategies of “homolog” (bold), “limited” (italic) and “soft” (gray) randomization, which allows wild type and homologous amino acids, limited diversity based on natural antibodies, or ~50% of wild type and 50% of all the other amino acids, respectively. The sequences of selected clones were aligned against E7 and mutations as shown. The relative affinity of each clone was assessed by phage IC50 as above. (B) IL5 as biotinylated protein binding to IL5 receptor coated on ELISA wells in the presence of buffer (PBS), or 50nM of E7 or two affinity improved variants (1C36, 1C60) of E7 was detected with streptavidin-HPR conjugate. (C) E7 and the improved variants (1C36 and 1C60) purified as Fabs were used as analyte in Biacore SPR measurements using a CM5 sensor chip immobilized with human IL5 (R&D Systems) or IL4 at 25 °C to determine the monovalent affinities.