Figures & data
Figure 1. Current and emerging routes of open innovation in the pharmaceutical industry. Besides the traditional open innovation approaches (in/out-licensing, co-development, co-commercialization, acquisition, spin-out/divestiture), the industry is selectively opening up boundaries and is exploring open innovation approaches to bring in new ideas and product opportunities to fill or enrich its pipe-line. Source: Joost Bakker (Scicomvisuals).
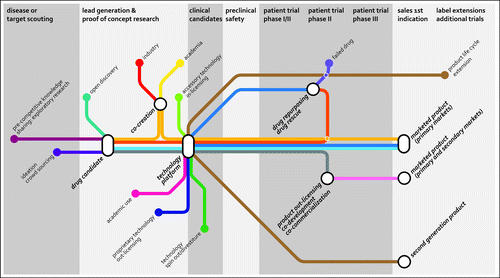
Figure 2. Science growing into applications. Insight in IgG4 biology and the unraveling of the IgG4 Fab-arm exchange process formed the roots for 3 major branches of research that led to the generation of scientific papers and intellectual property through various industry-academia collaborations. These culminated in 3 novel antibody technology platforms for the generation of stabilized IgG4, stable IgG4 half molecules (UniBody®) and bispecific IgG1 (DuoBody®). Numbers indicate: the number of collaborating parties involved; the number of patent applications filedCitation26,Citation27,Citation29-Citation35,Citation38-Citation41,Citation52; the number of scientific papers publishedCitation20-Citation25,Citation36,Citation37,Citation53-Citation62; and the number of platform licensees that occurred between 2003 (the beginning of the IgG4 project within Genmab) and April 2014. Source: Joost Bakker (Scicomvisuals).
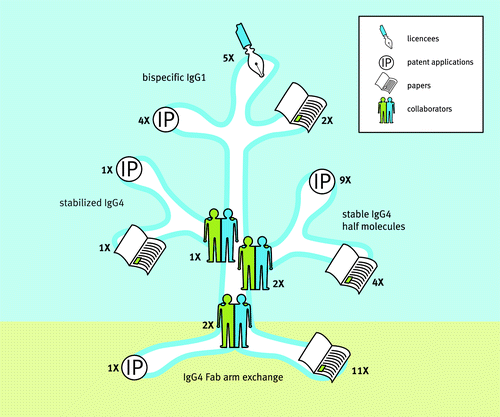
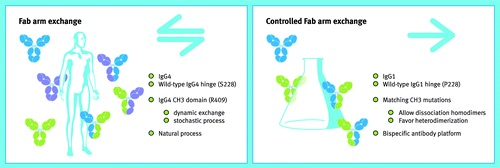
Figure 3. From nature to a bispecific technology platform. The left panel shows the major characteristics of the naturally occurring, bidirectional process of Fab-arm exchange as was discovered during the research described in this Perspective. This knowledge was applied to develop a platform of controlled, unidirectional Fab-arm exchange, of which the major characteristics are shown in the right panel. Source: Joost Bakker (Scicomvisuals).