Figures & data
Table 1. Monoclonal antibodies targeting human receptor tyrosine kinases that are currently approved in oncology
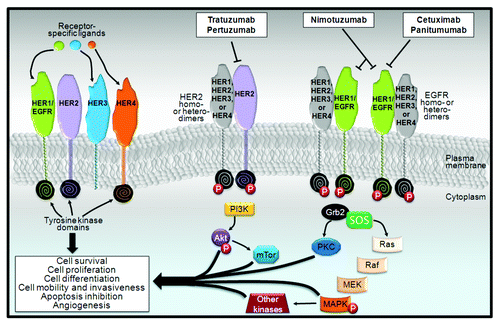
Figure 1. Overview of human epidermal growth factor receptor (HER) family signaling. Although the morphology of the extracellular domains of the four HERs are almost identical, their functional activity varies considerably. HER3 lacks inherent kinase function, but can heterodimerize with other HER receptors. Indeed, the HER2-HER3 dimer, which is considered to be the most active HER signaling dimer, is fundamental for HER2-mediated signaling in tumors with HER2 amplification. After ligand binding, the HER receptor becomes activated by dimerization between two identical receptors (i.e., homodimerization) or between different receptors of the same family (i.e., heterodimerization). Dimerization leads to the phosphorylation of several intracellular catalytic substrates, including members of the Ras/Raf/mitogen-activated protein kinase (MAPK) pathway, the phosphatidyl-3-kinase (PI3K)/Akt/PTEN family, and other important signaling pathways that regulate apoptosis, protein synthesis, and cellular proliferation. The monoclonal antibodies trastuzumab and pertuzumab are directed against HER2 members, with both leading to an inhibition of the HER2 signaling pathway but by two distinct mechanisms. Cetuximab and pertuzumab bind to the EGFR protein.