Figures & data
Figure 1. Dysregulated T cell function induced by CD28SA stimulation. (A) Human CD4+ TEMs were stimulated for 1 to 4 d with plate-bound anti-CD3 mAb (CD3, 5 μg/ml); NIB1412 (NIB1412, 10 μg/ml); anti-CD3 mAb and NIB1412 (CD3 and NIB1412); control category included cells without any treatment (Control). Cells were harvested at indicated time points and stained with fluorochrome-conjugated anti-CD4 and anti-TCR antibodies followed by flow cytometric analysis. Population of CD4+TCR+ cells are shown in the upper right quadrant as percentages of total T cells. Results are representative of four independent experiments. (B) Human CD4+ TEMs cells were stimulated with the indicated concentrations of plate-bound antibodies and proliferation was measured three days post-activation by 3H-labeled thymidine incorporation. The vertical axis represents mean cpm ± SD from triplicate wells. The data are representative of four independent experiments, (*P < 0.05, ***P < 0.001; unpaired t test). (C) Cell surface staining of human CD4+ TEMs stimulated for 4 d with 5 μg/ml of plate-bound anti-CD3 mAb and/or 10 μg/ml of NIB1412. The percentages of the CD4+ CD95+ cells are shown in the upper right quadrant. Results are representative of three independent experiments (D) Human CD4+ TEMs were stimulated for 1 to 6 d, fixed with ice cold 70% ethanol, stained with propidium iodide and cells in S-phase were quantified by flow cytometry. Results are representative of at least four independent experiments. (E) IL-2 concentrations in the supernatants from human CD4+ TEMs stimulated for 24, 48 and 72 h were determined by enzyme-linked immunosorbent assay (ELISA). The IL-2 titers from four independent experiments (mean ± SD of replicate samples) are expressed as picograms per mL on a log10 scale. (*p < 0.05, **p < 0.01, ***p < 0.001; two-way ANOVA).
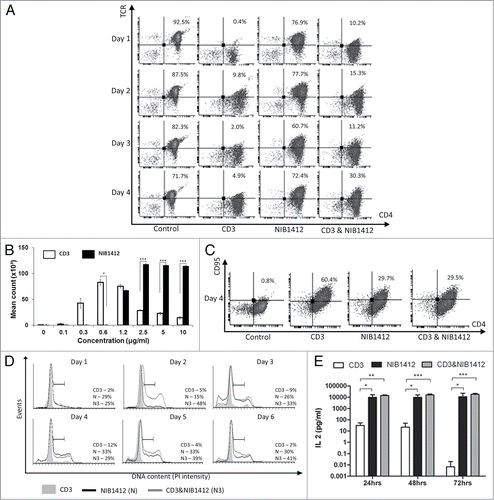
Figure 2. Enhanced cell surface expression of LFA-1 and CCR5 on CD28SA-activated CD4+ effector memory T cells. Human CD4+ TEMs were stimulated for 1 to 4 d with plate-bound anti-CD3 mAb (CD3, 5 μg/ml); NIB1412 (NIB1412, 10 μg/ml); anti-CD3 mAb and NIB1412 (CD3&NIB1412); control category included cells without any treatment (Control). Cells were harvested at indicated time points and stained with fluorochrome-conjugated anti-CD4 and anti-LFA (A) or anti-CD4 and anti-CCR5 (B) antibodies followed by flow cytometric analysis. (A) Population of CD4+LFA+ cells are shown in the upper right quadrant as percentages of total T cells. The cells are shown as percentages of total T cells in the upper right quadrant. (B) Population of CD4+CCR5+ cells are shown in the upper right quadrant as percentages of total T cells. Results are representative of four independent experiments.
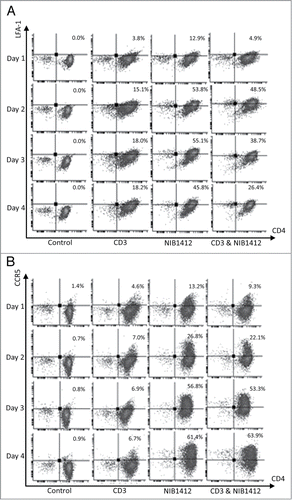
Figure 3. Adhesion and transmigration of CD28SA-activated CD4+ effector memory T cells. Human CD4+ TEMs were stimulated with plate-bound anti-CD3 mAb (CD3, 5 μg/ml); NIB1412 (NIB1412, 10 μg/ml); anti-CD3 mAb and NIB1412 (CD3 and NIB1412); control category included cells without any treatment (Control). Cells were harvested at 48 h and labeled with CellTracker™ Green CMFDA. (A) Immunofluorescent microscopy of HDMEC monolayer with adherent CMFDA-labeled TEMs initially stimulated with the indicated antibodies and stained for F-actin and DNA using fluorescently labeled phalloidin (red) and DAPI (blue), respectively. (Scale bar = 20 microns). Phase optics was adjusted to emphasize adherent cells. (B) CMFDA-labeled TEMs were added to confluent HDMECs and incubated for 30 min. Cells were washed and the remaining adherent cells visualized under fluorescence microscopy. Vertical axis represents the number of adherent TEMs per field (**p < 0.01; ***p < 0.001, unpaired t test). Results from four independent donors are represented as means ± SD (C) Transmigration of stimulated CD4+ TEMs across HDMEC layer on transwell inserts. Transmigrated cells were quantified by fluorescence and the results are presented as the mean (± SD) number of transmigrated cells from 3 replicate wells per condition. Results are representative of three independent experiments (***p < 0.001; unpaired t test).
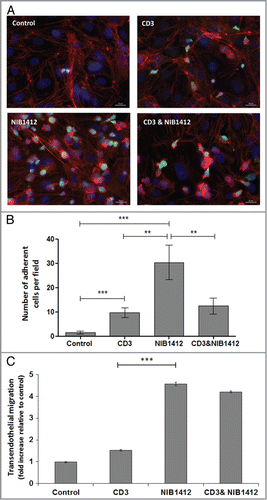
Figure 4. Failure of CD4+ effector memory T cells to upregulate cell surface PD-1 after CD28SA-activation. Human CD4+ TEMs were stimulated for 1 to 4 d with plate-bound anti-CD3 mAb (CD3, 5 μg/ml); NIB1412 (NIB1412, 10 μg/ml); anti-CD3 mAb and NIB1412 (CD3 and NIB1412); control category included cells without any treatment (Control). (A) Cells were harvested at indicated time points and stained with fluorochrome-conjugated anti-CD4 and anti-PD-1 antibodies followed by flow cytometric analysis. (B) Cells harvested at day 3 were stained for intracellular PD-1 following fixation/permeabilization treatment. Population of CD4+ PD-1+ cells are shown in the upper right quadrant as percentages of total T cells. Results are representative of four independent experiments.
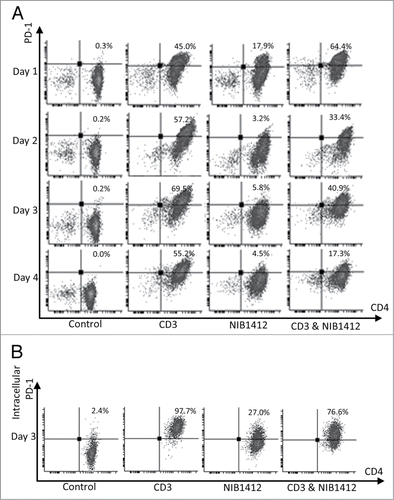
Figure 5. Absence of PD-1 mediated regulation of T cell function in CD28SA- stimulated T cells. (A) Schematic of the protocol used to investigate the functional significance of PD-1 pathway on NIB1412-activated CD4+ T cells. (B) Human PBMCs were stimulated for 48 h with plate-bound anti-CD3 mAb (CD3, 5 μg/ml); NIB1412 (NIB1412, 10 μg/ml); anti-CD3 mAb and NIB1412 (CD3 and NIB1412); control category included cells without any treatment (Control). Cells were then re-stimulated with anti-CD3 only (CD3,1 μg/ml) or with anti-CD3 and 10 μg/ml of rPD-L1 (CD3 and rPD-L1). Cells were harvested and stained with fluorochrome-conjugated anti-CD4 antibody, fixed, permeabilized, stained with fluorochrome-conjugated anti-IFNγ antibody and analyzed by flow cytometry. The CD4+ population is shown in light gray and the CD4− population in dark gray. The percentages of the CD4+ IFNγ+ cells are shown in the upper right quadrant. Results are representative of four independent experiments.
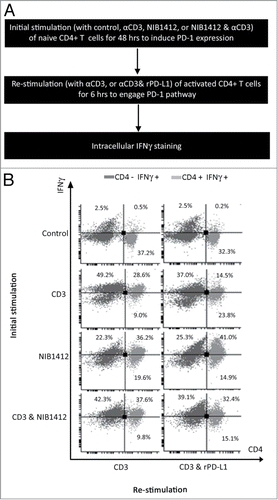
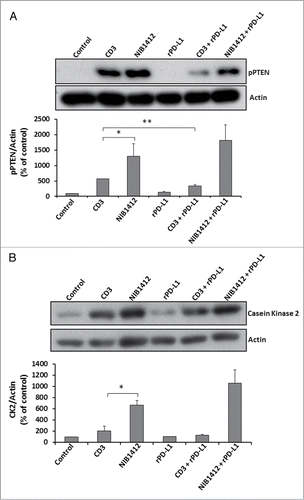
Figure 6. Effects of PD-1 engagement on phospho PTEN and casein kinase 2 levels in CD4+ effector memory T cells stimulated by anti-CD3 and CD28SA. Human CD4+ TEMs were stimulated with anti-CD3 mAb (CD3, 5 μg/ml); NIB1412 (NIB1412, 10 μg/ml); rPD-L1 (rPD-L1, 10μg/ml); with anti-CD3 mAb and rPD-L1 (CD3 + rPD-L1) or with NIB1412 and rPD-L1 (NIB1412 + rPD-L1). At the end of 72 h, the cells were lysed and extracted protein separated by SDS-PAGE. pPTEN – S380/T382/383 (A) and CK2 (B) protein levels were assessed by immunoblotting and β-actin was used as loading control. All blots are representative of four independent experiments. pPTEN and CK2 levels were quantified by densitometry, normalized to β-actin and expressed as a percentage of pPTEN or CK2 levels in untreated cells. Data are represented as mean ± SEM of four independent experiments. Statistical analysis was performed using unpaired t test (*p < 0.05, **p < 0.01). Phospho PTEN – pPTEN; Casein Kinase 2 – CK2