Figures & data
Figure 1 Schematic diagram and biochemical analysis of EI-04. (A) Schematic diagram of EI-04, composed of an anti-EGFR Fab linked to an effectorless human Fc with a stability-engineered anti-IGF-1R scFv attached at the C-terminus. (B) SDS-PAGE analysis of EI-04 under non-reducing (lane 2) and reducing (lane 3) conditions. (C) Analytical size exclusion chromatogram of EI-04. Static light scattering measurements indicate that the material is monomeric with MW ∼200 kDa. (D) Differential scanning calorimetry (DSC) analysis of EI-04. Raw data are shown as a solid black line, while the deconvoluted peaks, each representing an EI-04 domain (Fab, CH2, CH3, scFv), are shown as dotted lines. The deduced domain assignments are indicated above each peak.
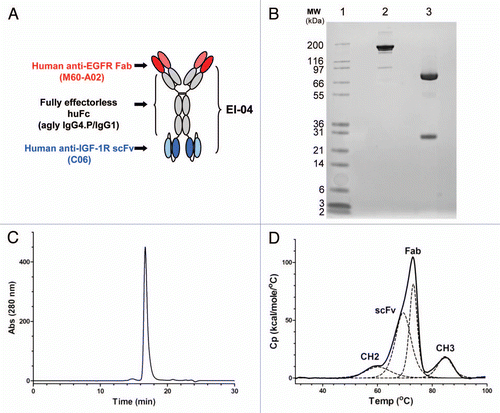
Figure 2 Binding activities of EI-04 BsAb and its parental anti-EGFR and anti-IGF-1R mAbs. (A and B) Biacore surface plasmon resonance sensorgrams of soluble EGFR ectodomain (0–30 nM) to chip-captured EI-04 (A) and anti-EGFR mAb M60-A02 (B). (C) Octet Red biolayer interferometry-based equilibrium analysis for soluble IGF-1R ectodomain binding to tip-bound EI-04 and anti-IGF-1R mAb C06. (D) Biacore surface plasmon resonance sensorgrams demonstrating serial binding of IGF-1R ectodomain and Fc-tagged EGFR ectodomain to EI-04.
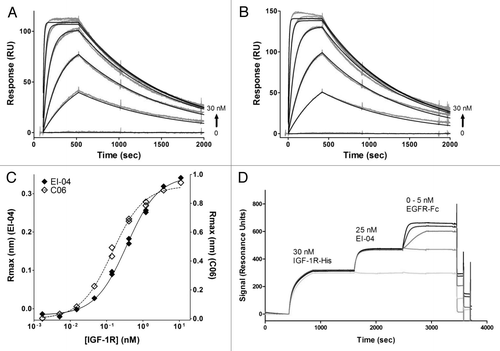
Figure 3 Dual inhibitory activities of EI-04 on EGFR and IGF-1R. (A) Blockade of europium-labeled EGF binding to EGFR-Fc by EI-04 in comparison to various anti-EGFR mAbs. (B) Blockade of a constant level of IGF-1 (25 nM, left part) or IGF-2 (60 nM, right part) binding to biotin-hIGF-1R-Fc by EI-04 in comparison to anti-IGF-1R mAb C06. (C) Inhibition of EGF-induced EGFR phosphorylation in H322M tumor cells by EI-04 in comparison to anti-EGFR mAb M60-A02. Serum-starved cells were treated with EI-04 or M60-A02, or control IgG (ctrl Ab) for 1 h followed by stimulation with 100 ng/ml of EGF for 15 min. Phospho-EGFR and total EGFR were analyzed by western blot. (D) Inhibition of IGF-1-induced IGF-1R phosphorylation in H322M tumor cells by EI-04 in comparison to anti-IGF-1R mAb C06. Serum-starved cells were treated with EI-04 or C06 or control IgG (ctrl Ab) for 1 h followed by stimulation with 200 ng/ml of IGF-1 for 20 min. Phospho-IGF-1R and total IGF-1R were analyzed by western blot.
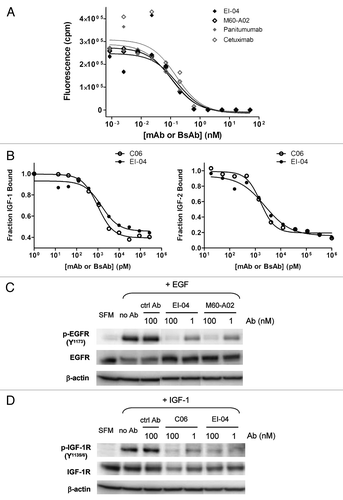
Figure 4 Concurrent blockade of EGFR and IGF-1R signaling pathways and enhanced inhibition of tumor cell growth and cell cycle progression by EI-04. (A) Simultaneous inhibition of phosphorylation of EGFR and IGF-1R in HN11 tumor cells by EI-04. Cells grown in culture medium supplemented with 10% FBS were treated with the indicated antibodies against EGFR and IGF-1R or a control IgG (ctrl Ab) for 4 h. Phospho-EGFR, phospho-IGF-1R, total EGFR, total IGF-1R and β-actin in cell lysates were analyzed by western blot. (B) Simultaneous blockade of phosphorylation of Akt and ERK in HN11 cells by EI-04. Cells were treated as described in (A). Phospho-Akt (left part) and phospho-ERK (right part) levels were quantified using MSD. Data are means ± SD (n = 2), representative results from two similar experiments. (C) Improved inhibition of serum-driven HN11 cell growth by EI-04 compared to single mAbs. Tumor cells grown in culture medium supplemented with 10% FBS were treated with serially diluted antibodies starting from 300 nM for three days prior to cell viability determination. Percent growth inhibition was calculated relative to no antibody treatment control. Data are means ± SD (n = 3), representative results from two similar experiments. Significance of difference between mAbs and EI-04: *p < 0.05, **p < 0.01, ***p < 0.001, by one-way ANOVA. (D) Enhanced blockade of serum-driven HN11 cell cycle progression by EI-04 compared to single mAbs. Tumor cells were grown in serum-free medium (SFM) or in culture medium supplemented with 10% FBS and treated with 100 nM of the indicated antibodies against EGFR and IGF-1R or a control IgG (ctrl Ab) for two days. Percentage of cells in each cell cycle phases was determined by FACS analysis.
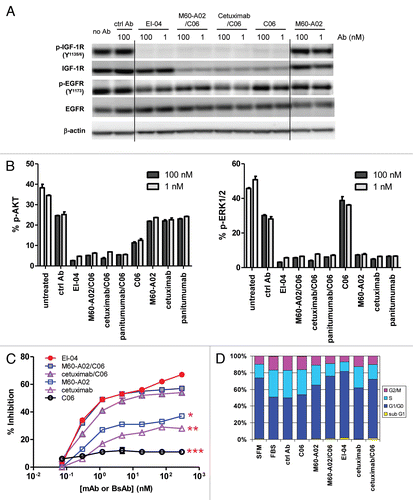
Figure 5 High avidity binding and synergistic inhibitory effect of EI-04 on BxPC3 tumor cells. (A) Flow cytometric measurement of the abilities of EI-04, M60-A02 and C06 to compete with Alexa 647-labeled C06 (left part) and Alexa 647-labeled M60-A02 (right part) for binding to BxPC3 tumor cells. Mean fluorescence intensity was measured when cells were stained with Alexa 647-C06 or Alexa 647-M60-A02 in the presence of various serially diluted competitor antibodies. Data are representative results from two similar experiments. (B) Inhibition of IGF-1-stimulated (left part) or EGF-stimulated (right part) BxPC3 tumor cell growth by EI-04, M60-A02, C06 or the M60-A02/C06 combination. Cells grown in serum-free medium supplemented with 100 ng/ml of IGF-1 or EGF were treated with serially diluted antibodies for three days prior to cell viability determination. Percent growth inhibition was calculated relative to no antibody treatment controls. Data are means ± SD (n = 3), representative results from two similar experiments. (C) Inhibition of BxPC3 cell colony formation by EI-04, M60-A02, C06 or the M60-A02/C06 combination. BxPC3 cells plated in soft agar were treated with a single dose of 0.3 or 100 nM of the indicated antibodies and allowed to grow in complete medium supplemented with 100 ng/ml of EGF and IGF-1 each for two weeks. Fluorescence signals correlating with live colonies are shown. Data are means ± SD (n = 4), representative results from two similar experiments.
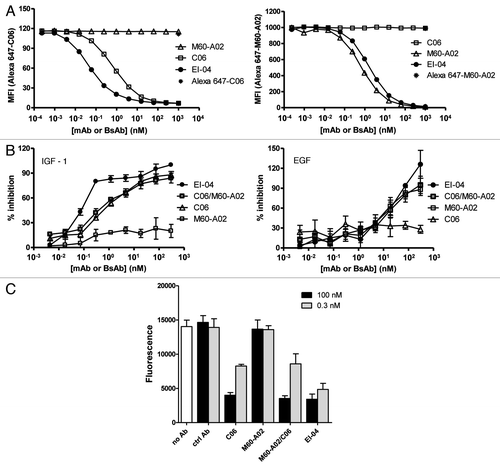
Figure 6 Comparable pharmacokinetic profiles of EI-04 and M60-A02 in nude mice. The serum concentrations of EI-04 and M60-A02 are shown for the indicated time points after a single tail vein injection of each antibody at 5 or 20 mg/kg. PK parameters from WinNonLin analysis are indicated in the bottom table.
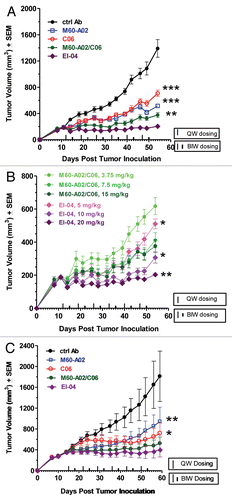
Figure 7 In vivo superior efficacy of EI-04 in two xenograft models. (A) Efficacy of EI-04 at 20 mg/kg in comparison to M60-A02 or C06 dosed at 15 mg/kg as single agents or in combination in the BxPC3 pancreatic cancer model. (B) Efficacy of EI-04 at 20 mg/kg, 10 mg/kg and 5 mg/kg in comparison to the M60-A02/C06 combination dosed at 15 + 15 mg/kg, 7.5 + 7.5 mg/kg and 3.75 + 3.75 mg/kg in the BxPC3 pancreatic cancer model. (C) Efficacy of EI-04 at 20 mg/kg in comparison to M60-A02 or C06 dosed at 15 mg/kg as single agents or in combination in the GEO colon cancer model. Tumor-bearing mice were given intraperitoneal administration of a control IgG (ctrl Ab, 5C8), M60-A02 and EI-04 twice a week (BIW) or C06 once a week (QW). The dosing schedules are indicated on the x-axis. The mean tumor volumes from each treatment group of approximately 10 mice were plotted as a function of time. The differences in the tumor growth rate between the mAb treatment groups and the EI-04 group dosed at the equal molar doses were indicated by *p < 0.05, **p < 0.01 or ***p < 0.001, if they are statistically significant as determined by one-way ANOVA.