Figures & data
Figure 1 The schematic depicts the general structure of a DVD-Ig protein where CH and VH refer to constant and variable heavy chain regions, respectively, and CL and VL refer to constant and variable light chain regions, respectively. CD and VD refer to constant domain and variable domain, respectively. The variable regions form tandem inner and outer variable domains, connected by both light and heavy chain linkers, on each arm of the DVD.
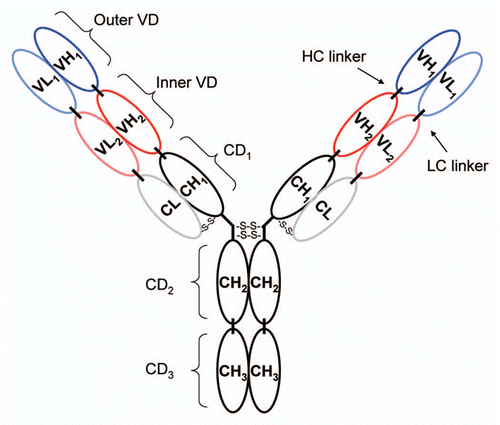
Figure 2 Cleavage of DVD-03, DVD-04 and DVD-05 (referred to simply as 03, 04 and 05) with entorokinase or thrombin visualized by reducing SDS-PAGE. (A) The DVD-Ig proteins before (−) and after (+) cleavage with either enterokinase or thrombin. (B) The cleaved DVD-Ig proteins after purification on Mab Select SuRe columns. HC and LC refer to the heavy and light chains, respectively. Frag 1 refers to the cleavage product of the light chain consisting of the constant region and the inner variable region; Frag 2 refers to the cleavage product of the light chain consisting of the outer variable region.
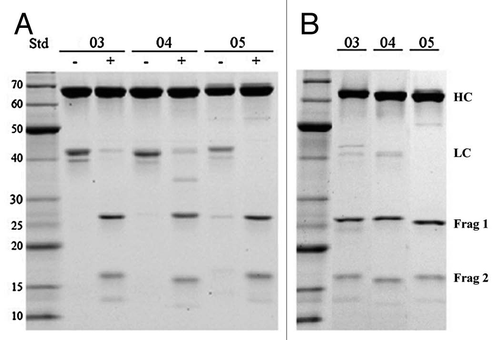
Figure 3 Papain cleavage can be used to generate functional fragments from DVD-Ig proteins. (A) Papain can cleave a DVD-Ig protein between CH1 and CH2 resulting in a product denoted as a DFab that contains intact outer and inner variable domains. (B) Papain cleavage of DVD-02 resulted in an additional secondary product, denoted DFab(48k) where papain cleavage also occurred in both heavy and light chain linkers between the inner and outer variable domains.
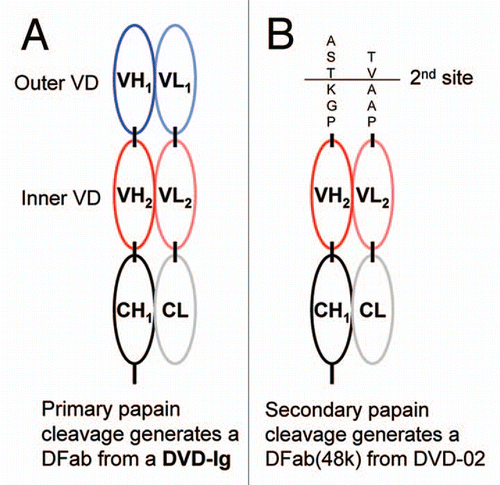
Figure 4 ka vs. kd plot derived from data presented in for TNFα binding kinetics of the intact reference mAb and DVD-Ig proteins. Diagonals show affinities (KD); the vertical and horizontal dashed lines intersect at the reference mAb kinetic constants.
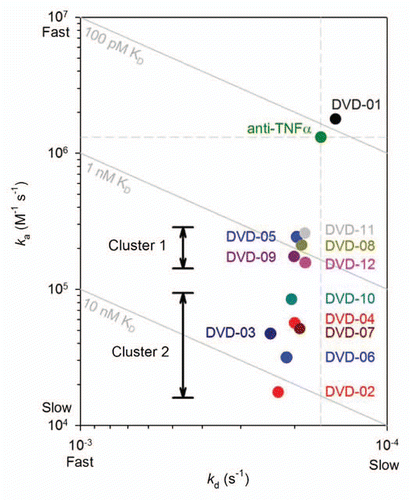
Figure 5 ka vs. kd plot derived from data presented in for TNFα binding kinetics of the intact reference mAb and DVD-03, DVD-04 and DVD-05 before (solid circles) and after (empty circles) cleavage of the light chain linkers with either enterokinase or thrombin proteases. Diagonals show affinities (KD); the vertical and horizontal dashed lines intersect at the reference mAb kinetic constants.
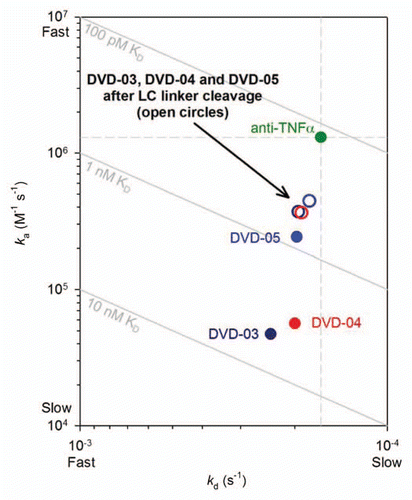
Figure 6 ka vs. kd plot derived from data presented in for TNFα binding kinetics (anti-human(H+L) assay format). Solid circles are the intact reference mAb, DVD-01 and DVD-02; empty circles are the reference Fab, DVD-02-DFab and the DVD-02-DFab(48k). The red arrow highlights the difference in binding kinetics for the DVD-02-DFab and the DVD-02-DFab(48k). Diagonals show affinities (KD); the vertical and horizontal dashed lines intersect at the reference mAb kinetic constants when assayed via anti-human (Fc).
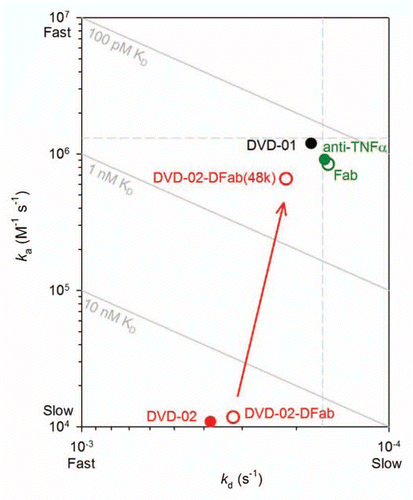
Table 1 DVD-Ig protein identities
Table 2A TNFα binding kinetics (via anti-hu Fc)
Table 2B TNFα binding kinetics (via anti-hu H+L)
Table 3 DVD-Ig protein affinity clusters