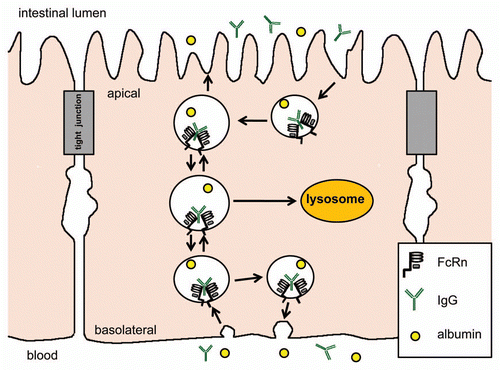
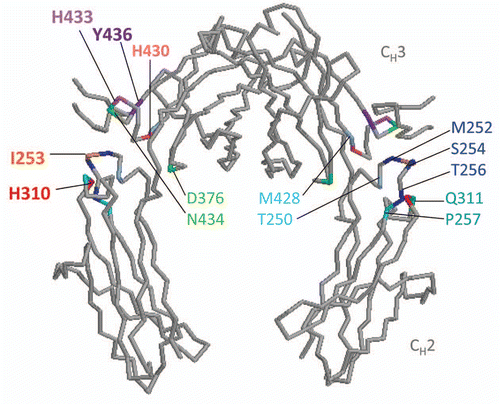
Figures & data
Table 1 A summary of IgG mutations developed to increase FcRn-IgG binding affinity and extend serum half-life