Figures & data
Figure 1 Serum CMAB007 concentration-time curve in healthy, male Chinese subjects after single SC administration of different doses. CMAB007 150 mg (□); CMAB007 300 mg (●); CMAB007 600 mg (△) (n = 9 for each dose group). Data were expressed as mean ± SD.
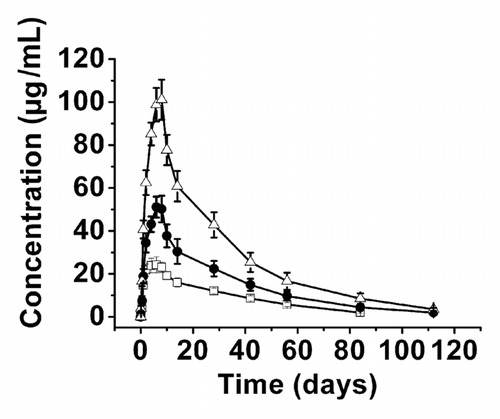
Figure 2 Linear regression analyses of (A) AUC0-∞ (R2, 0.9997) and (B) Cmax (R2, 0.9979) of CMAB007 after single SC administration. Individual value (○).
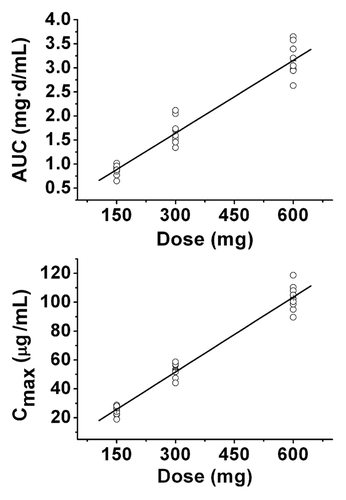
Figure 3 Serum CMAB007 concentration-time curve in healthy, male Chinese subjects following multiple SC doses of 150 mg q4wk × 6 (□, n = 3) and 300 mg q4wk × 6 (●, n = 5). Data were expressed as mean ± SD.
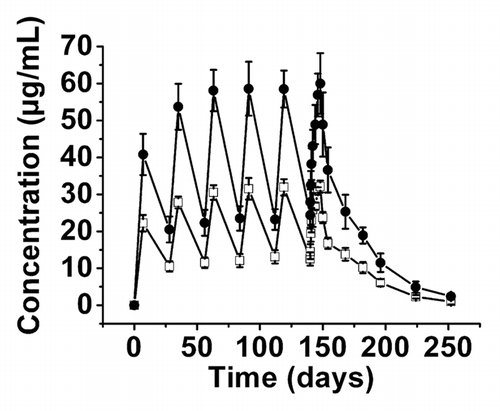
Figure 4 Serum CMAB007 concentration-time curve in healthy, male Chinese subjects following single and multiple doses of 150 mg and 300 mg. Single-dose of 150 mg (○, n = 9); multiple-dose of 150 mg (●, n = 3); single-dose of 300 mg (△, n = 9); multiple-dose of 300 mg (▴, n = 5). Data were expressed as mean values.
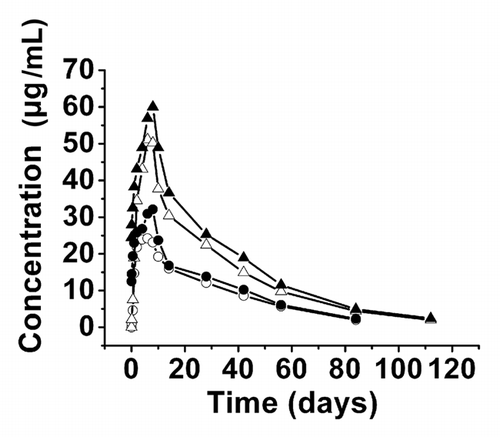
Figure 5 Serum concentration-time curve of (A) total IgE and (B) free IgE after single SC administration of different doses. CMAB007 150 mg (□); CMAB007 300 mg (●); CMAB007 600 mg (△) (n = 9 for each dose group). Data were expressed as mean ± SD.
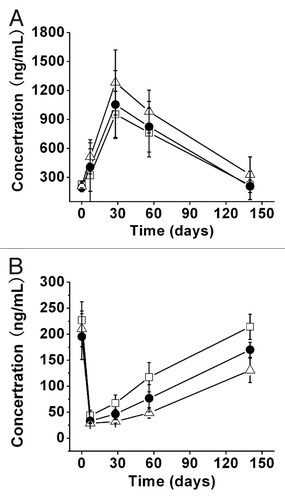
Table 1 Demographic characteristics of subjects enrolled in single- and multiple-dose studies
Table 2 Treatment-emergent adverse events reported during single- and multiple-dose administration of CMAB007
Table 3 Noncompartmental and one-compartmental pharmacokinetic parameters of CMAB007 after administration of single doses in healthy Chinese subjects
Table 4 Noncompartmental and one-compartmental of individual pharmacokinetic parameters of CMAB007 at steady-state in healthy Chinese subjects
Table 5 PK/PD of CMAB007 vs. Xolair®