Figures & data
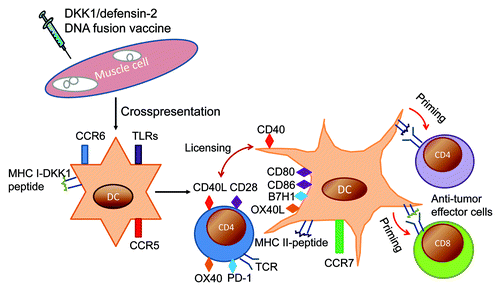
Figure 1. Proposed schema of anti-tumor immunity following DNA vaccination with DKK1/defensin-2 DNA fusion vaccine. Following intramuscular injection in mice of DKK1/defensin-2 DNA fusion vaccine, muscle and resident APCs are transfected with plasmid, leading to fusion protein production. Murine defensin-2 plays a role in activating APCs via CCL-6 and TLR4. Cross-priming occurs in which CD8+ T cell responses are primed by exogenous MHC Class I–restricted antigens that are not expressed in, but rather are acquired by, local APCs. These DCs (dendritic cells) acquire antigen, express CCR7, and are attracted by chemokines expressed in the draining lymph nodes, where they prime naive T cells. DKK1 is processed, and peptides are presented by MHC Class I molecules; this peptide/MHC Class I complex stimulates CD8+ T lymphocytes. Soluble protein released by transfected cells is taken up by DCs, and via the MHC Class II pathway, the peptide/MHC Class II complex stimulates CD4+ T lymphocytes, and MHC Class II-binding peptides from DKK1 activate the large repertoire of anti-DKK1 CD4+ T cells. Pairs of receptor–ligands (matched colors) interact to 'license' the DCs to maintain presentation of tumor-derived peptides that are able to prime anti-tumor CD8+ and CD4+ T cells. CpGs interact with TLR9 in APCs, where they induce the expression of costimulatory molecules such as CD80 and CD86, MHC Class II molecules, and pro-inflammatory cytokines. B7H1-blocking antibodies interrupt T-cell immunosuppression through PD-1/B7 family signaling. OX40-agonist antibodies might affect the roles of Treg cells through OX40 signaling since mouse Treg cells constitutively express OX40. Therefore, DKK1 DNA vaccine plus CpG combined with B7H1-blocking or OX40-agonist antibodies could break the immunosuppression of T-cell response and lead to much better anti-tumor immunity induced by the vaccine. TCR, T-cell receptor.