Figures & data
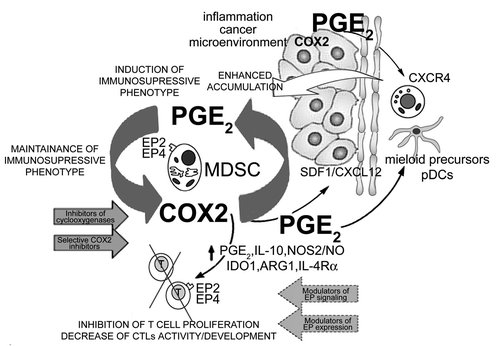
Figure 1. Positive COX2-PGE2-EP2/EP4-mediated feedback loop in the biology of cancer-associated MDSCs. Inflammation (IL-1β, TLR ligands, IFN-γ) and/or cancer-produced PGE2 or PGE2 inducers drive the early induction of COX2 in local myeloid cells (monocytes, macrophages, immature DCs), promoting their production of suppressive factors (IDO1, IL-10, ARG1, NOS2 and PGE2 itself), and acquisition of suppressive functions. The EP2- and EP4-dependent signals are also critical in the induction and persistence of functional CXCR4 on monocytic cells and for the production of CXCL12/SDF-1 in cancer environment. These processes are further amplified by the de novo-produced endogenous PGE2, now produced at high levels by MDSCs themselves, thereby creating a positive feedback loop, leading to accumulation of MDSCs in cancer environment. In addition to inducing other suppressive factors, PGE2 also directly suppresses CTL development and functions, acting via EP2 and EP4 receptors. The key role of the EP2- and EP4-mediated COX2-PGE2 feedback to control multiple aspects of MDSC function provides for convenient targets to control MDSC-associated immune dysfunction in cancer immunotherapy.