Figures & data
Figure 1.ADA expression and function in CD4+CD39+ Treg vs. CD4+CD39neg Teff. (A) PBMC obtained from NC were stained with relevant antibodies and analyzed by flow cytometry. The % expression of FOXP3 and CD26 on CD4+CD39+ T cells is shown. Representative dot plots were selected from 15 independent experiments performed. (B) Single cell-sorted CD4+CD39+ and CD4+CD39neg cells were stained for CD39, ADA, CD26 and DAPI. Expression and co-expression of these markers were analyzed by confocal microscopy (mag x 400). Results of one out of five representative experiments are shown. (C) Single cell-sorted CD4+CD39+ and CD4+CD39neg cells were plated in 96-well plates (25,000 cells/well) in serum-free medium with 10 µM of exogenous adenosine and were incubated for different time periods. Levels of adenosine remaining in the cell supernatant (i.e., un-metabolized adenosine) were determined by mass spectrometry. Data are from an experiment representative of three performed with cells of different donors. (D) Single cell-sorted CD4+CD39+ and CD4+CD39neg cells were plated in 96-well plates (25,000 cells/well) with 10 µM of exogenous adenosine in the presence or absence of different inhibitors. Adenosine levels remaining in the cell supernatants were determined 20 min later by mass spectrometry. Data (means ± SD) are from three indepent experiments. Asterisks indicate p < 0.05 for differences between CD4+CD39+ and CD4+CD39neg cells.
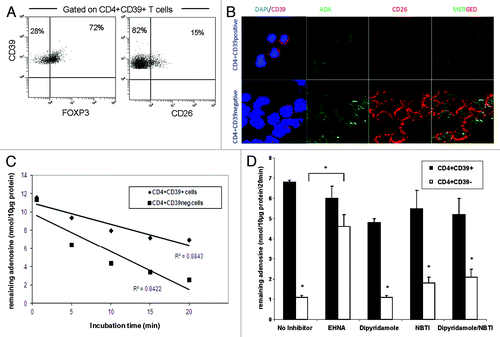
Table 1. Gene expression of CD26 and ADA in CD4+CD39+ and CD4+CD39neg T cells subsets
Figure 2.Frequency and activity of CD4+CD39+CD26neg in NC and HNSCC patients. (A) Freshly isolated PBMC from NC (n = 15) and HNSCC patients (n = 15) were stained and analyzed by flow cytometry. The data are means ± SD. The asterisk indicates p < 0.01. (B) PBMC obtained from NC or HNSCC patients were single-cell sorted into CD4+CD39+CD26neg and CD4+CD39neg responder T cells (RC). The latter were CFSE labeled and stimulated with plate-bound OKT-3 and soluble anti-CD28. Following addition of CD4+CD39+CD26neg suppressor cells and 150 IU/mL of IL-2, the co-cultures were incubated for 5 d. Cells were analyzed by flow cytometry as described in Materials and Methods. Suppression of RC cell proliferation mediated by CD4+CD39+CD26neg obtained from NC or cancer patients at various S/RC ratios is shown. Data represent means ± SD from three independent experiments. (C) Tumor-infiltrating T cells are shown in sections of a representative HNSCC of five specimens examined (mag. x 400) (1) tumor cells (red, stained with pancytokeratin) (2) few green CD26+ T cells are among tumor cells; tumor cell nuclei are white (DAPI); no ADA+ cells stained blue are visible. (3) Sections 1 and 2 in a merged image. Tumor cells are negative for ADA and CD26. (4) A lymphoid infiltrate into tumor is stained for CD4 (green), ADA (red) and CD26 (blue). A co-localization of ADA and CD26 in CD4+ cells (pink) is evident. (5) A tumor section containing infiltrating lymphoid cells is stained for CD4 (green), ADA (red) and FOXP3 (blue). CD4+FOXP3+ Treg (green/blue) are negative for ADA, whereas CD4+FOXP3neg Teff cells (yellow/red) are positive for ADA. (6) A lymphoid infiltrate into tumor is stained for CD4 (green), ADA (blue) and CD132 (red). CD4+CD132+ Tr1 are negative for ADA (yellow/red), whereas CD4+CD132neg Teff cells are mostly positive for ADA (blue).
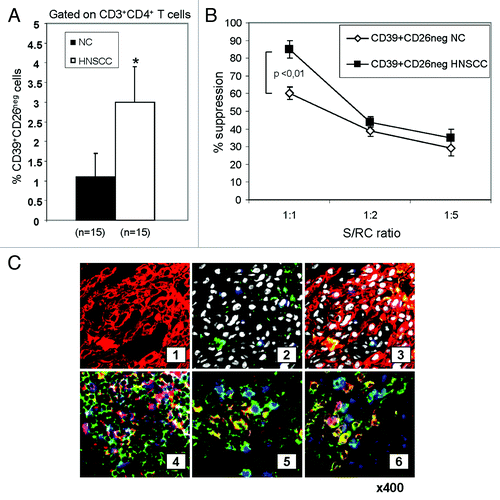
Figure 3. ADA activity in Teff of NC and patients with HNSCC. (A) Freshly isolated PBMC from NC (n = 15) and HNSCC patients (n = 15) were stained and analyzed by flow cytometry for percentages of CD39negCD4+CD26+ T cells. The data are means ± SD. The asterisk indicates p < 0.05. (B) Single cell-sorted CD4+CD39neg cells from NC and HNSCC patients were plated in 96-well plates (25,000 cells/well) with 10 µM of exogenous adenosine and in the presence or absence of different reagents. Remaining adenosine levels were determined in the cell supernatants after 20 min of addition of exogenous adenosine by mass spectrometry. Data (means ± SD) are from three independent experiments per group. Asterisks indicate p < 0.05. (C) PBMC obtained from a representative NC or HNSCC patient were single-cell sorted into CD4+CD39+ (S) and CD4+CD39neg T cells (RC). The latter were CFSE labeled and stimulated with plate-bound OKT-3 and soluble anti-CD28. Afterwards S were added to the culture and incubated with 150 IU/ml of IL-2 for 5 d. Cells were analyzed by flow cytometry as described in Materials and Methods. To selected wells CADO (20 μM) was added. The FACS plots are from an experiment representative of three independent experiments performed. (D) Results of suppression assays performed as outlined in C. The data are means ± SD. The asterisk indicates p < 0.01. (E) Single cell-sorted CD4+CD39neg cells were plated in 24-well plates (1x106 cells/well) and activated with OKT-3 (1 μg/ml) and anti-CD28 (1 μg/ml) in the presence or absence of CADO (20 μM) for 24 h. Cytokine levels in the cell supernatants were determined by LUMINEX. Data (means ± SD) are from five independent experiments. Asterisks indicate p < 0.05
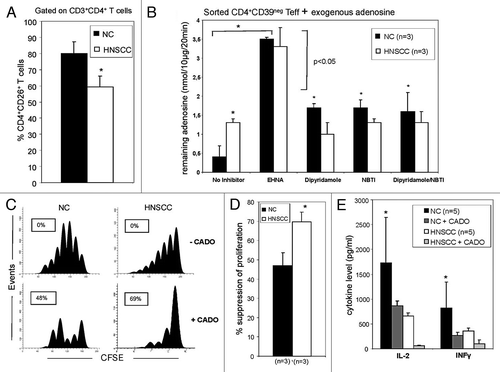
Figure 4.ADA expression in CD4+CD39neg Teff in NC and HNSCC patients. (A) PBMC obtained from NC and HNSCC patients were stained with relevant antibodies and analyzed by flow cytometry. The % surface and intracellular expression of ADA in CD4+CD39neg T cells is shown. Data represents the mean and standard deviation from five independent experiments per group performed. Asterisks indicate p < 0.05 for differences between NC and HNSCC patients. (B) The mean fluorescence intensity of surface and intracellular ADA expression in CD4+CD39neg T cells is evaluated by flow cytometry. Data represents the mean and standard deviation from five independent experiments performed. Asterisks indicate p < 0.05 for differences between NC and HNSCC patients. (C) CD4+CD39neg T cells were treated with various cytokines and factors for 48 h and afterwards evaluated by flow cytometry for their % surface expression of ADA. Data represents the mean and standard deviation from five independent experiments performed. Asterisks indicate p < 0.05 for differences compared with the baseline value.
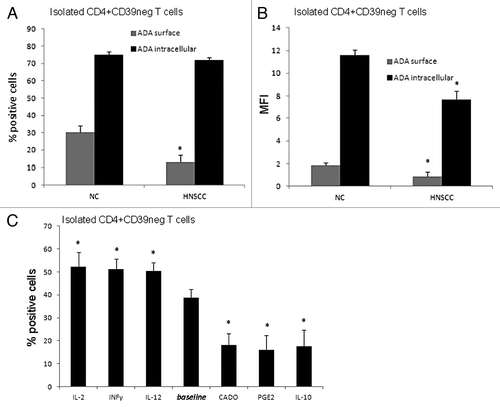
Table 2. Influence of different cytokines on adenosine deaminase activity of Teff cells
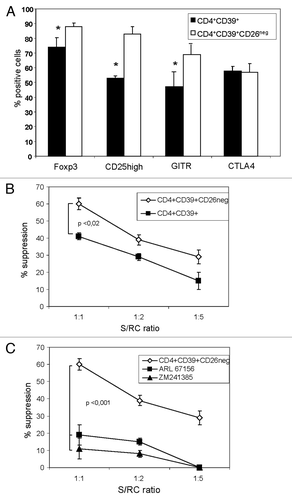
Figure 5.The phenotype and suppression mediated by CD4+CD39+ Treg vs. CD3+CD39+CD26neg Treg. (A) Freshly sorted PBMC obtained from 15 NC were stained and analyzed by flow cytometry. Expression of conventional Treg markers in the CD4+CD39+ and CD4+CD39+CD26neg T cell subsets was determined. Data are means ± SD from 15 independent experiments. (B) Single-cell sorted CD4+CD39neg cells were CFSE-labeled and stimulated with plate-bound OKT-3 and soluble anti-CD28 in the presence of CD4+CD39+ or CD4+CD39+CD26neg suppressor cells and 150 IU/ml of IL-2 for 5 d. The % inhibition of RC proliferation was determined by flow cytometry and analyzed using the Modfit software. (C) An inhibitor of CD39 activity (ARL67156) or an antagonist of the A2AR (ZM 241385) were added to the suppression assays at the beginning of the co-cultures set up as described in (B). Suppression of CD4+CD39neg cell proliferation mediated by CD4+CD39+ cells or CD4+CD39+CD26neg cells at various S/RC ratios was determined as described in Materials and Methods. The data are means ± SD from three independent experiments.