Figures & data
Figure 1. Vaccine-specific HLA-DQ4 and –DP4 restricted CD4+ T-cell clones display high affinity for GV1001 peptide but also recognize a nested 9-mer epitope. The relative peptide affinity of GV1001-specific T cell clones was tested in proliferation assays against titrated peptide concentrations from 10−5-10−10 M (A). Open labels show HLA-DP4 restricted T cell clones and closed labels show HLA-DQ4 restricted T cell clones. (B) shows the relative affinity of HLA-DQ4 restricted T-cell clones for GV1001 compared with nested 9-mer 618–626 tested in proliferation assays. Open labels show T-cell clones tested against GV1001 peptide and closed labels show T cell clones tested against peptide 618–626.
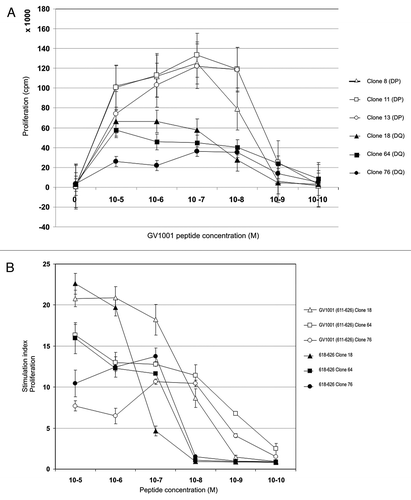
Figure 2. Cytotoxic CD4+ CTL clones specific for GV1001 lyse peptide-loaded target cells most efficiently with nested 9-mer epitope. Both HLA-DP and –DQ restricted T-cell clones recognize processed hTERT protein. GV1001-specific T-cell clones were also tested for proliferation against EBV-LCL loaded with recombinant hTERT protein at low concentrations (A). One representative HLA-DP restricted clone is shown (black bars) and one HLA-DQ restricted clone (hatched bars). (B) shows 8- and 24-h 51Cr-release assays for HLA-DQ restricted CTL clones tested against autologous EBV-LCL loaded with peptides GV1001 (611–626) and nested epitope 618–626. Peptide 617–625 was used as a negative control. Effector: Target ratio was 100:1. Black bars represent clone 18, dotted bars clone 64, and hatched bars clone 76.
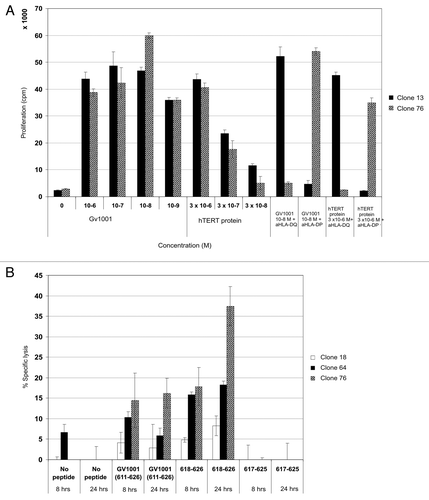
Figure 3. CD4+ CTL clones express granzyme B, TRAIL, TNF-α and low levels of CD107a, but not perforin. Expression of Granzyme B, perforin, TRAIL, CD107a, IFN-γ, TNF-α and IL-2 was measured by flow cytometry after overnight incubation of T cell clones with peptide GV1001- loaded autologous EBV-LCL. Effector:target ratio was 1:3. Top panels in A-D show T-cell clone 64 and bottom panels show T-cell clone 76. Left panels show responses against non-peptide loaded controls and right panels show GV1001 peptide-loaded target cells.
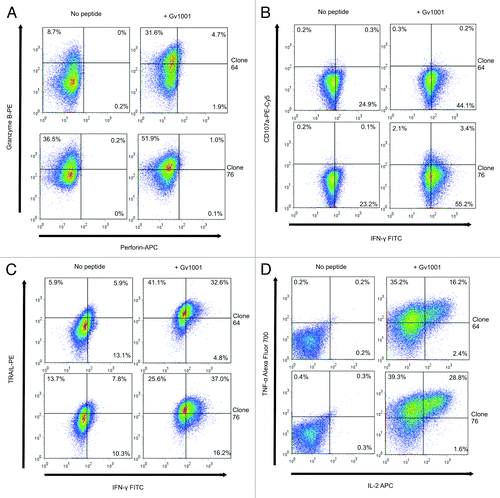
Figure 4.T-cell reactivity against multiple unrelated hTERT Th and CTL epitopes. (A) shows pentamer analysis of hTERT-specific HLA-B*0702 restricted CD8+ T cells post vaccination. PBMCs were stained ex vivo with two pentamers with novel hTERT peptides 613–621 and 672–681 which were nested epitopes of GV1001 and 660–689, respectively. Vaccination started in 2002 and post vaccination samples from the years indicated were tested. Black bars represent T cells specific for peptide 613–621-specific, dotted bars peptide 672–681 and hatched bars the HIV control peptide. (B) shows a summary of all post-vaccination-T cell responses detected against several unrelated 15- and 30-mer hTERT peptides. PBMCs from different post-vaccination sample time points were pre-stimulated with 25 overlapping hTERT peptides and tested for proliferation against single peptides.
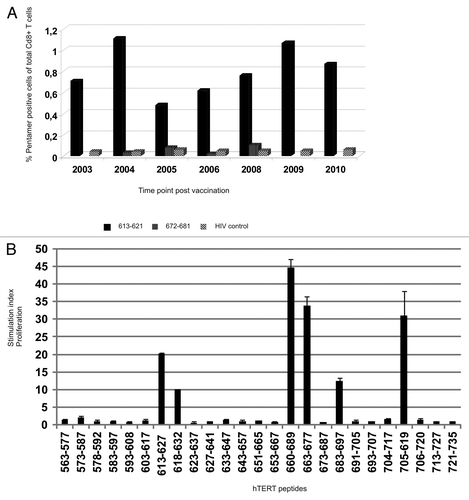
Figure 5. Peptide-specific T-cell proliferation correlates with IFN-γ secretion and give rise to high affinity CD4+ T-cell clones. (A) shows hTERT-specific T-cell proliferation and IFN-γ production in response to peptide-loaded autologous PBMC. The x-axis shows peptide, the Y axis proliferation measured in cpm and the z-axis shows number of spots detected per 105 T cells in an IFN-γ ELISPOT on the same cells. White dotted bars represent proliferation of 613–621-stimulated T cells, black dotted bars the proliferation of GV1001-stimulated T cells, and small-dotted bars 660–689-stimulated T cells, respectively. Large hatched bars represent the IFN-γ secretion from 613–621-stimulated T cells, small hatched bars the GV1001-stimulated T cells and black bars the 660–689-stimulated T cells, respectively. (B) shows proliferation of three representative CD4+ T-cell clones against autologus EBV-LCL loaded with titrated concentrations of peptide 660–689. Closed squares show T cell clone 57, open squares show T cell clone 107 and closed triangles show T cell clone 109.
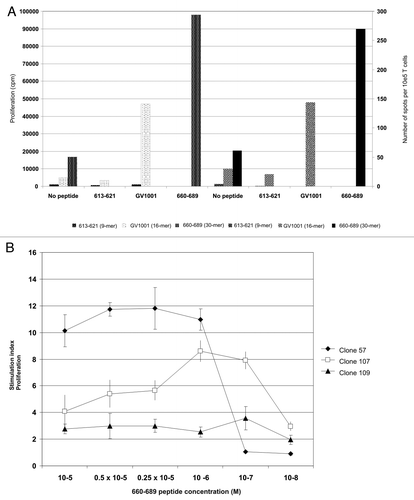
Figure 6. Multifunctional HLA-DR*08 restricted CD4+ T cell clones specific for novel hTERT epitope display an inflammatory cytokine profile and early effector memory phenotype. (A) shows intracellular cytokine staining of three representative HLA-DR*08 restricted CD4+ T cell clones upon overnight stimulation with hTERT 30-mer peptide 719–20-loaded autologus EBV-LCL. Clones were tested for production of cytokines IL-2, IFN-γ and TNF-α. Non-peptide loaded EBV-LCLs were used as negative control. (B) and (C) show the T-cell phenotype. T cells were tested for CD45RA and CD45RO expression (B) and CCR7, CD28, CD27, CD62L, CD57 (C). Grey lines represent no peptide (A) or isotype controls (C), blue lines clone 57 (A and C), black lines clone 107 (A and C) and brown lines clone 109 (A and C).
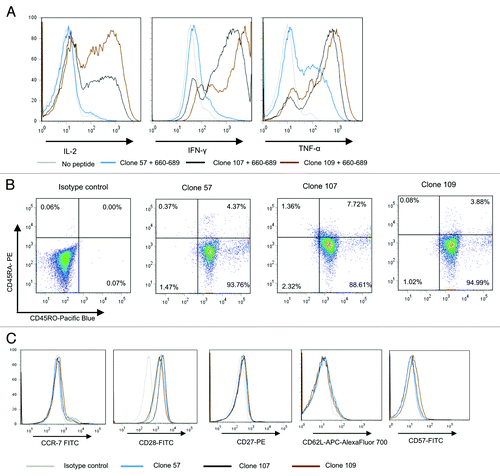
Figure 7. HLA-DR*0801 restricted CD4+ T cell clone specific for novel hTERT epitope recognizes melanoma cell line. Intracellular cytokine staining of the hTERT-specific HLA-DR*08 restricted CD4+ T cell clone 109 was performed after overnight stimulation with autologus EBV-LCL or the HLA-DR*0801 melanoma cell line, ESTDAB-100. As positive controls, the EBV-LCL and ESTDAB-100 were both loaded with hTERT 30-mer peptide 660–689.
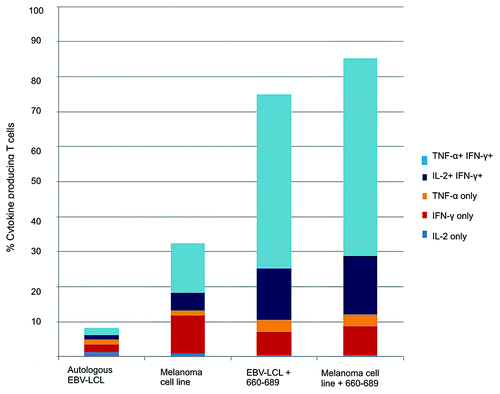
Figure 8. T-cell reactivity against multiple unrelated hTERT Th and CTL epitopes in several long-term surviving GV1001-vaccinated patients. PBMCs from different post-vaccination sample time points were pre-stimulated with 25 overlapping hTERT peptides and tested for proliferation against single peptides. The graphs show a summary of post-vaccination-T cell responses detected against several unrelated 15- and 30-mer hTERT peptides. Two melanoma patients (A and B) and one colon cancer patient (C) were tested.
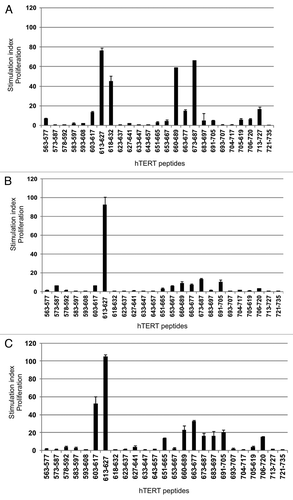
Figure 9. T-cell reactivity against hTERT in short-term surviving GV1001-vaccinated patients is less broad. PBMCs from post-vaccination sample time points were pre-stimulated with 6 hTERT peptides from our test panel frequently inducing responses in long-term survivors. T-cells were then tested for proliferation against single peptides. The graph shows a summary of post-vaccination-T cell responses detected against the different hTERT peptides. Six melanoma patients and four lung cancer patients were tested.* indicates the GV1001 peptide sequence (611–626).
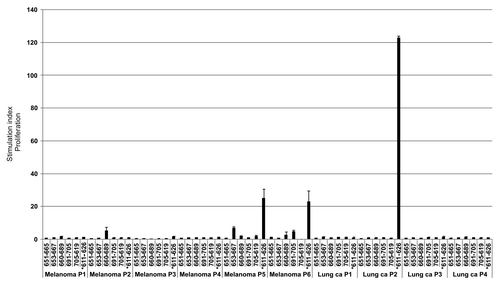
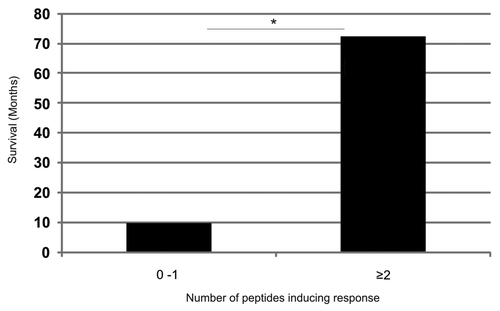
Figure 10. Increased survival correlates with the recognition of an increased number of additional hTERT peptides in patients responding immunologically against the GV1001vaccine. Five of the most frequently recognized hTERT peptides from our overlapping peptide library (653–667, 651–665, 660–689, 691–705, 705–719) were tested for their capacity to stimulate T cells in ten melanoma and lung cancer patients who had responded immunologically against the GV1001 vaccine but displayed below median survival. The T-cell responses were compared with those of four clinical responders/long-term survivors who also responded immunologically against the GV1001 vaccine. Patients were grouped according to whether they recognized 0–1 peptides (n = 10) or ≥ 2 peptides (n = 4) in addition to GV1001 with a median survival of 10 mo and 72.5 mo, respectively. The Mann-Whitney U test was used for testing the correlation between survival and T-cell responses against an increased number of peptides.*p = 0.005