Figures & data
Figure 1. Ex vivo analysis of urine T cells during BCG therapy. (A) Mean +/− SEM of the absolute number of total cells recovered from urine samples before (white square) and after (black square) each BCG instillation (n = 11 patients). (B) Representative example of ex vivo CD3, CD8 and CD4 staining of urine cells from one NMIBC patient before and after the sixth BCG treatment. CD8 and CD4 dot plots are gated on CD3+ population. (C) Mean +/− SEM of absolute number of CD3 T cells from the analyzed urine samples before (white square) and after (black square) each BCG instillation (n = 9 patients).
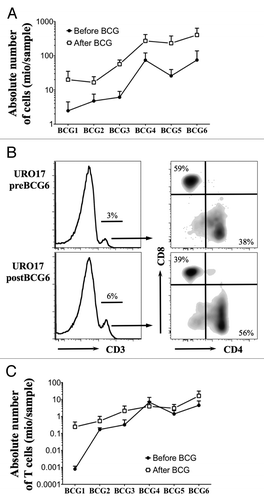
Table 1. Expansion of cells from urine of NMIBC patients. Cells from available urine samples were stimulated in presence of allogenic irradiated PBMC, 150 U/ml IL-2 and 1 µg/ml PHA, either directly after washing (n = 7 patients) or after CD3+ magnetic sorting (n = 6 patients)
Figure 2. Analysis of urine T cells expanded in vitro. (A and B) Percentage of CD3+, CD3+CD8+ and CD3+CD4+ T cells after in vitro amplification from total urine cells (A) or magnetically isolated T lymphocytes (B). (C) CD8/CD4 ratio among CD3+ T cells from ex vivo samples (vertical axis) were plotted against those obtained after in vitro amplification of CD3+ sorted T cells (horizontal axis). Spearman correlation R and p values are indicated.
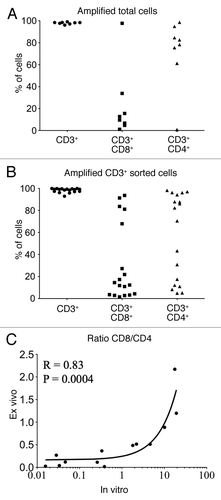
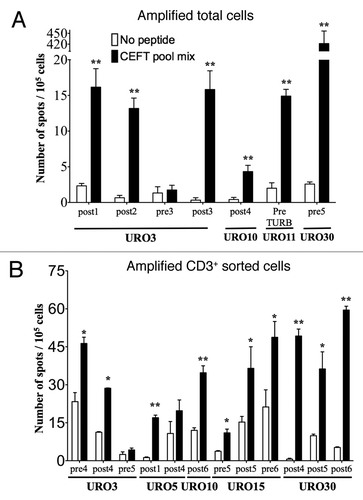
Figure 3. Detection of antigen-specific T cells in the urine of NMIBC patients. The presence of functional antigen-specific T lymphocytes was assessed using an IFNγ- specific ELISPOT assay on amplified total cells (A) or amplified CD3+ sorted cells (B) as isolated from the urine of NMIBC patients. Samples from each of the indicated URO patient correspond to urines obtained at the specified pre- and/or post-BCG instillation or pre-TURB time, after which T cells were expanded in vitro and tested by ELISPOT. Number of IFNγ secreting cells (spot)/105 cells are indicated in presence of peptide (black bars) or medium alone (white bars). Significant differences were calculated with the free website tool (http://www.scharp.org/zoe/runDFR).Citation17 p < 0.05 and p < 0.01 are indicated by * and **, respectively.